Composition characteristics of gases separated continuously from manufactured exhalated gas scattered in water using hollow fibers
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In this study, manufactured exhaled gas was mixed with water using a hollow fiber membrane, and the possibility of underwater breathing using the separated gases was investigated through another hollow fiber membrane. As the amount of mixed water increased, the amount of carbon dioxide in the separated gas decreased. Water was supplied at a rate increasing from 10 LPM to 30 LPM, and manufactured exhaled gas was introduced at a rate increasing from 1.2 LPM to 6.3 LPM. When water was supplied at 30 LPM and exhaled air was introduced at 6.3 LPM, the amount of carbon dioxide in the separated gas was 0.75%. This study demonstrates that by continuously processing manufactured exhaled gas underwater, the separated gas can be used in real-time for underwater breathing.
Keywords:
Hollow fibers, Carbon dioxide, Scattered, Manufactured exhalated gas, Composition1. Introduction
When a material with a hydrophobic surface that allows the passage of gases is used, the permeation characteristics of gases due to concentration differences have been reported [1]. If a hollow box made of such a material is submerged in water, gas movement occurs depending on the difference in gas concentration between the outside and inside of the box. If a battery that consumes oxygen is placed inside a box that does not have gas permeation characteristics, turning on the switch will result in a gradual decrease in oxygen concentration over time. However, if the same battery is placed in a box made of a hydrophobic, gas-permeable material, the oxygen concentration inside the box will initially decrease and then gradually reach equilibrium. In other words, as oxygen is consumed inside the box, oxygen from the water, where the concentration is relatively higher, permeates into the box, where the concentration is lower. This means that the oxygen concentration inside the box decreases initially but then stabilizes. The amount of oxygen consumed inside the box is replenished by the oxygen from the surrounding water that permeates through the box. The oxygen consumption rate inside the box due to the battery resembles the oxygen consumption rate of a human. Considering this result, if the surface area of the box is sufficiently large, enough oxygen to match the human consumption rate can be supplied from the water into the box. A box with a large surface area and these characteristics suggests the possibility of human respiration underwater.
When an empty box is submerged in water, the oxygen concentration inside the box is affected by the box's permeability, surface area, thickness, and partial pressure [3]. These correlations have been presented in equations and supported experimentally. In the case of the same material, it can be observed that an increase in thickness leads to a decrease in the change in oxygen concentration, while an increase in the contact area of the boundary layer where oxygen and water meet leads to an increase in the change in oxygen concentration.
The geometric characteristics of body surface hair affecting the formation of air layers underwater have been reported [3]. Insects, for example, have hairs on their bodies that help form an air layer underwater. The air layer allows them to intake oxygen for respiration and release carbon dioxide. If the spacing between the hairs is narrow, the air layer remains stable even as water pressure increases with depth. However, if the spacing between the hairs increases, the air layer becomes more prone to collapse under higher water pressure. Therefore, factors such as hair spacing, diameter, and contact angle influence the maintenance of the air layer at different depths. It has been shown that when an air layer is maintained around a body with hair underwater, respiration becomes possible through this layer.
For humans to breathe underwater, an air tank is necessary. However, since air tanks have a limited capacity, they restrict the duration of underwater breathing. With the increasing need for underwater rescue operations, exploration, and construction, the duration of underwater activities has also increased. Thus, examples of effectively securing the necessary amount of air for underwater breathing have been reported.
One such example involves utilizing exhaled air and reducing the carbon dioxide contained in it. Studies have shown that when exhaled air is well mixed with water, the amount of air available for breathing increases [4]. When exhaled air is broken into smaller sizes and mixed with water, the amount of carbon dioxide in the separated gas decreases. A motor is used to break down the exhaled air into smaller sizes, but the use of a motor increases the required power, thus reducing the time available for underwater breathing.
To reduce power consumption, a case has been reported where a manual mixer was used to decrease the amount of carbon dioxide in exhaled air [5]. The structure involved mixing water and exhaled air by passing them through several plates with small holes in the flow path, improving the mixing process. The resulting gas separated from the mixed water showed a reduced amount of carbon dioxide. This demonstrates that the amount of carbon dioxide in exhaled air can be reduced without using a motor to break the air into smaller sizes. However, the small holes in the plates increased flow resistance. As a result, the pressure drop required to supply the mixed water to the separation device increased, leading to a greater energy demand.
In this study, hollow fiber membranes were used to effectively mix exhaled air and water. By using bundles of hollow fibers, the exhaled air was broken down into smaller sizes before mixing, increasing the contact surface area with the water. When exhaled air is injected into the individual hollow fibers within the bundles, the air is mixed with the water through the gaps in the hollow fibers. After the finely broken exhaled air is mixed with water and flows into the next hollow fiber membrane, a vacuum pump is used to separate the gas through the interior of the hollow fibers. The supply of water was increased to 10, 20, and 30 LPM, while the flow of exhaled air was increased in five stages from 1.2 LPM to 6.3 LPM. As the amount of mixed exhaled air increased, the amount of carbon dioxide in the separated gas also slightly increased. When the supply of water was increased, the amount of carbon dioxide in the separated gas decreased. Generally, if the concentration of carbon dioxide is less than 1%, the gas can be used for breathing. The experimental results showed that as the concentration of carbon dioxide in the separated gas decreased, it became more promising for use in underwater breathing.
2. Experimental Methods
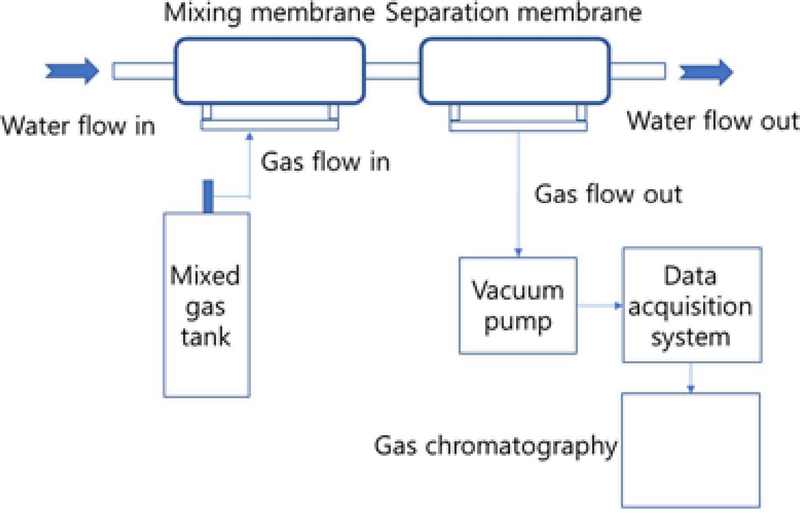
The experimental setup, as shown in Figure 1, consists of an exhaled gas chamber, two hollow fiber membranes, a vacuum pump, a data acquisition system, and a gas chromatography unit. Manufactured exhaled gas was prepared, taking into account the characteristics of human exhalation. The gas chromatography model iGC7200 made from DS SCIENCE was used. Gas chromatography was performed by injecting a 5cc sample, and the concentrations of oxygen, nitrogen, and carbon dioxide were measured.The hollow fiber membrane used was a 4 x 13 membrane contactor from LiquiCell.
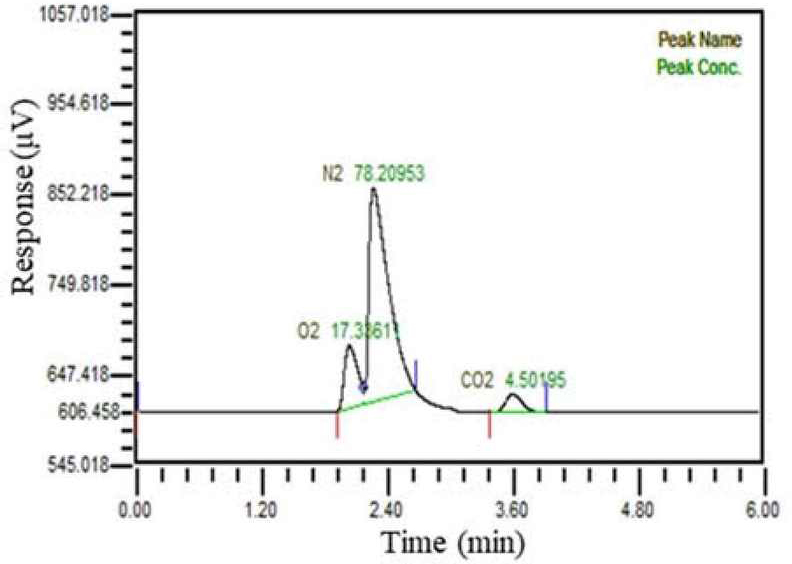
Among the two hollow fiber membranes, the first membrane was used for mixing the exhaled gas with water, while the second membrane was employed to separate gases from the water that contained the exhaled gas. The gases separated through the second hollow fiber membrane were sampled in a sampling bag and analyzed using gas chromatography to determine their composition. The pressure generated by the vacuum pump was measured by the data acquisition system. Figure 2 showed composition of manufactured gas.
The supplied water was controlled by adjusting the valve using the water pressure provided by the laboratory. Since the manufactured exhaled gas during experiment had a composition similar to that of typical exhaled gas, there were no particular issues, and proper ventilation was ensured as needed. For a safe experiment, it is necessary to conduct a preliminary test to check for potential issues related to leaks at the connections between experimental devices before carrying out the main experiment.
3. Results and Discussion
The hollow fiber membrane used can supply up to 40 LPM of water due to structural factors. Typically, exhaled gas is produced at a rate of 5-7 LPM. If a smaller amount of exhaled gas is used, the concentration of processed carbon dioxide is lower, but in order to increase the necessary air volume, it is important to process as much exhaled gas as possible. However, processing a larger volume of exhaled gas increases the amount of carbon dioxide contained in the treated gas, so the maximum possible exhaled gas is taken into consideration. Based on that, the water supply rate and experimental conditions for the produced gas were set.
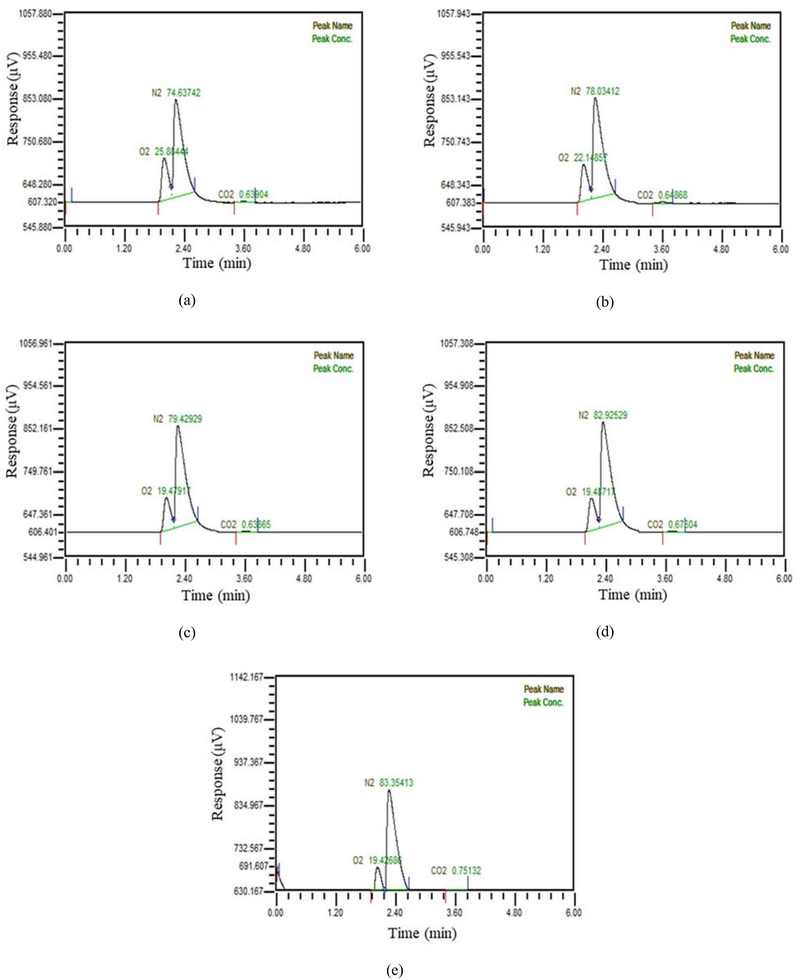
In Figure 3, when water was supplied at 10 LPM, the manufactured exhaled gas was supplied at increasing rates in five stages from 1.2 LPM to 6.3 LPM and mixed in a first hollow fiber membrane. The gases were separated and sampled in a sampling bad using a vacuum pump connected to the second hollow fiber membrane. The amount of carbon dioxide in the separated gas was measured using a gas chromatography. Figure 3 showed the concentration of carbon dioxide in gas separated from water which included 2 LPM exhalated gas was found to be less than 1%. Concentration of carbon dioxide in gas separated from water which included 3 LPM exhalated gas was found to be more than 1%.
Composition of gas separated at flow rate of 10 LPM when a) 1.2 LPM of manufacture exhaled gas is mixed b) 2.4 LPM of manufacture exhaled gas is mixed c) 3.6 LPM of manufacture exhaled gas is mixed d) 5 LPM of manufacture exhaled gas is mixed e) 6.3 LPM of manufacture exhaled gas is mixed
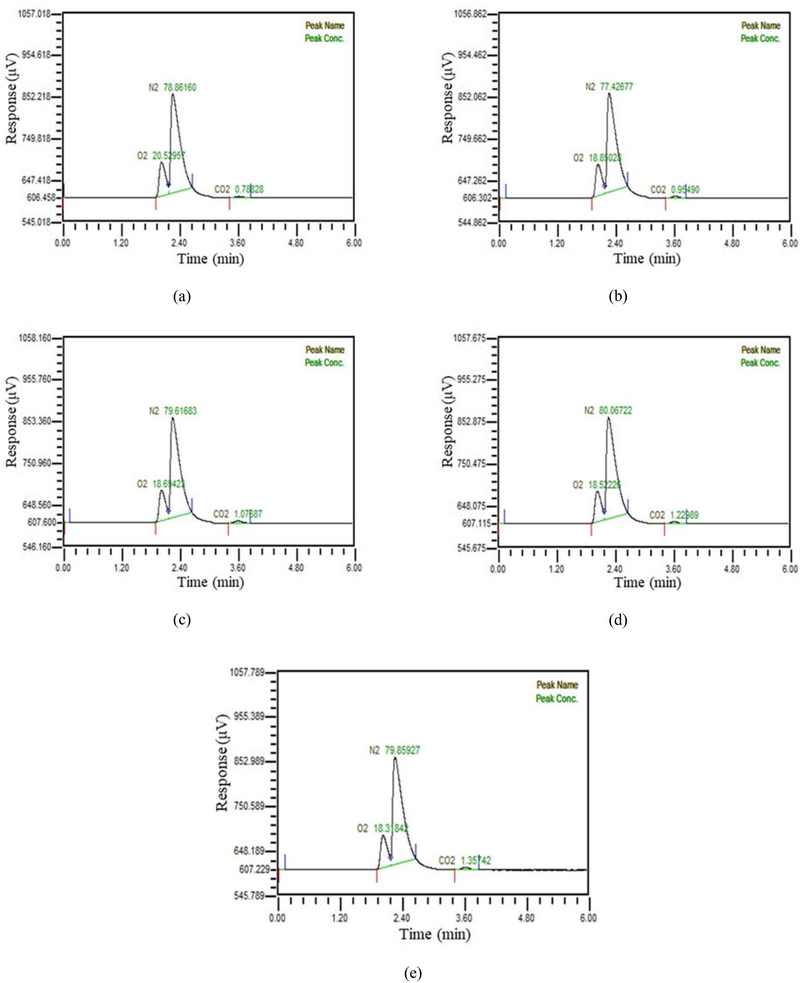
In Figure 4, when water was supplied at 20 LPM, the manufactured exhaled gas was similarly supplied at increasing rates in five stages from 1.2 LPM to 6.3 LPM and mixed in a first one. The amount of carbon dioxide in the separated gas was also less than 1%. Compared to the condition with water supplied at 10 LPM, the amount of carbon dioxide separated in condition with water supplied at 20 LPM was observed to decrease. It was considered more water was easy for manufacture gas to contact with water.
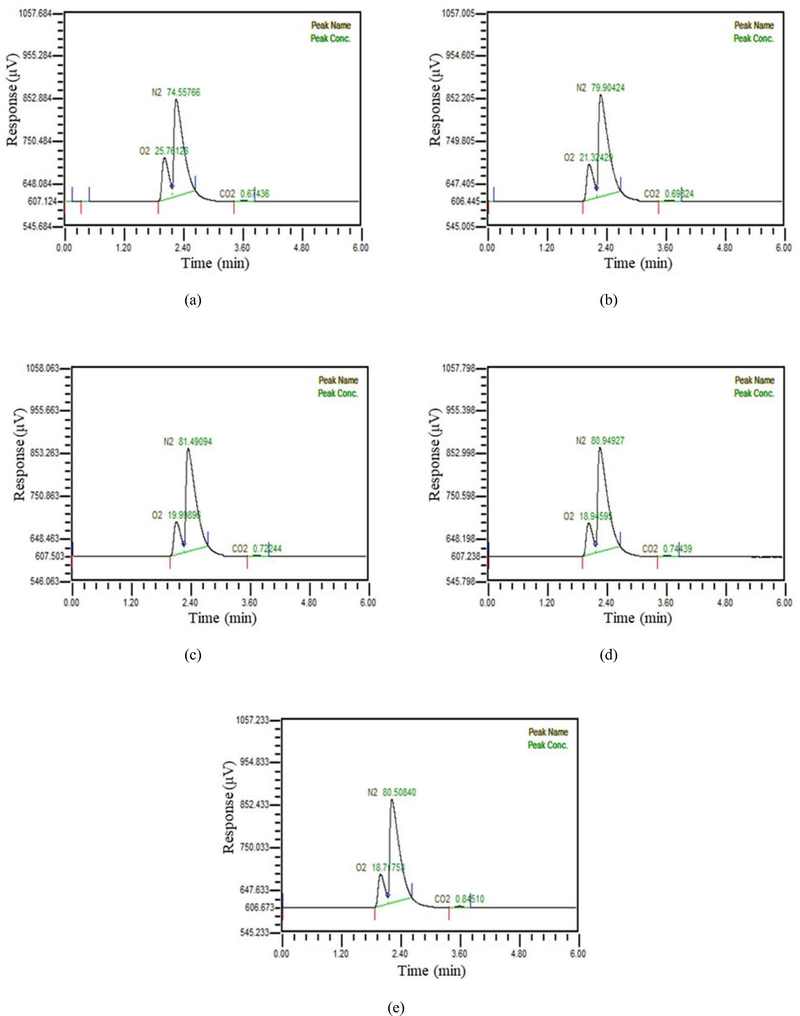
Composition of gas separated at flow rate of 20 LPM when a) 1.2 LPM of manufacture exhaled gas is mixed b) 2.4 LPM of manufacture exhaled gas is mixed c) 3.6 LPM of manufacture exhaled gas is mixed d) 5 LPM of manufacture exhaled gas is mixed e) 6.3 LPM of manufacture exhaled gas is mixed
In Figure 5, when water was supplied at 30 LPM, the amount of manufactured exhaled gas was increased in five stages from 1.2 LPM to 6.3 LPM, and the composition of the separated gas was analyzed using the gas chromatography. As the amount of manufactured exhaled gas increased, the amount of separated carbon dioxide also increased. However, as the water supply increased to 30 LPM, the amount of carbon dioxide in the separated gas decreased, and it was observed to remain below 1%.
4. Conclusion
This paper presents the possibility of using exhaled gas for underwater breathing after reducing the carbon dioxide content. By improving the mixing between the incoming water and exhaled gas, the amount of carbon dioxide in the gas separated from the mixed water was also reduced. Bundles of hollow fiber membranes were used, with exhaled gas supplied through the inside of the hollow fibers and water flowing around the outside. After effective mixing of the supplied exhaled gas and water, the mixture was passed through a separation hollow fiber membrane. The results demonstrated a reduction in the amount of carbon dioxide in the separated gas. Additionally, as the volume of supplied water increased, the amount of carbon dioxide in the separated
gas further decreased. This study was conducted at atmospheric pressure and room temperature. In order to apply it in underwater conditions, additional experiments considering various pressure conditions and temperatures need to be executed.
Acknowledgments
This research was supported by a research project (Development of Underwater Breathing Device Technology without An Oxygen Tank, 20026073) the Korea Planning & Evaluation Institute of Industrial Technology (KEIT) under the Korea government Ministry of Trade, Industry and Energy
Author Contributions
Conceptualization, Methodology, Software, Formal Analysis, Investigation, Resources, Data Curation, Writing-Original Draft Preparation, Writing-Review & Editing, Visualization, Supervision, Project Administration, Funding Acquisition, P. W. Heo.
References
-
N. J. Shirtcliffe, G. McHale, M.I. Newton, C.C. Perry and F.B. Pyatt, “Plastron properties of a superhydrophobic surface,” Applied Physics Letters, vol. 89, no. 10, 104106, 2006.
[https://doi.org/10.1063/1.2347266]

-
M. R. Flynn and J.W.M. Bush, “Underwater breathing: The mechanics of plastron respiration,” Journal of Fluid Mechanics, vol. 608, pp. 275-296, 2008.
[https://doi.org/10.1017/S0022112008002048]

-
J. Lee, P. W. Heo, and T. Kim, “Theoretical model and experimental validation for underwater oxygen extraction for realizing artificial gills,” Sensors and Actuators A: Physical, vol. 284, pp. 103-111, 2018.
[https://doi.org/10.1016/j.sna.2018.09.071]

-
P. W. Heo, “Characteristics of mixed gases separated from water including small sized bubbles scattered by motor rotation,” Journal of the Korean Society of Marine Engineering, vol. 43, no. 9 pp. 768-772, 2019 (in Korean).
[https://doi.org/10.5916/jkosme.2019.43.9.768]

-
P.W. Heo, “Composition of mixed gas with exhalation characteristics separated by dissolved gas separator with multi-stage passive mixer,” Journal of Advanced Marine Engineering and Technology, vol. 46, no. 5, pp. 270-276, 2022.
[https://doi.org/10.5916/jamet.2022.46.5.270]