Corrosion evaluation of a newly developed high-strength steel in marine environments
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study aims to evaluate the corrosion behavior of a newly developed high-strength steel in marine environments. Metals used in seawater are easily deteriorated because of the presence of corrosive species such as chloride ions in it. Seawater causes much higher corrosion than fresh water. Thus, the corrosion of steel in marine environment has been recognized as a crucial problem in designing structures which cannot be cathodically protected. In this study, the corrosion resistance of a newly developed high-strength steel was evaluated. Four different specimens were tested to confirm the corrosion resistance. The exposure corrosion test was carried out by exposing the specimens to different marine environments such as atmospheric, tidal, splash, and submerged zones for two years. The specimens taken out from each location were cleaned ultrasonically and chemically prior to the evaluation of their corrosion resistance by the weight loss method. Finally, the pitting depth of the specimens was also measured to evaluate their pitting corrosion. The conditions used for the corrosion test were similar to the environmental conditions. The corrosion test results revealed that the corrosion rate and pitting corrosion of the newly developed high-strength steel was lower than that of the other carbon steels.
Keywords:
Chloride ions, Corrosion resistance, Weight loss, Pitting, High-Strength steel1. Introduction
Steels are easily deteriorated in seawater because the ions such as sodium, chlorine, and sulfide present in it accelerate the corrosion reaction. The electrical conductivity of seawater is 400–4000 times higher than that of fresh water depending on the environment. In marine environment, steel structures are exposed its atmospheric, splash, tidal, or submerged zones. The corrosion rate and corrosion behavior of steel structures depend upon the marine environment exposure conditions.
Many studies have been carried out on the corrosion behavior (under various environmental conditions), lifetime extension, and improvement of the corrosion resistance and pitting resistance of high-strength steels [1].
Feng et al. evaluated the electrochemical corrosion of an ultrahigh-strength carbon steel in H2S-containing alkaline brines using in situ electrochemical techniques. They found that the corrosion rate decreased at higher pH values and increased at higher H2S concentrations. The effect of the H2S concentration on the corrosion rate of the steel varied as the pH varied. The corrosion products changed from iron carbonate and sulfides to iron oxides as the pH increased [2]. Guo et al. simulated the stress corrosion cracking behavior of a high-strength casing steel in air. They found that the stress corrosion cracking tendency and stress corrosion cracking susceptibility of the steel increased with an increase in the temperature [3]. Park et al. investigated the effect of the type and size of inclusions on the pitting corrosion behavior of dual-phase, transformation-induced plasticity, and twinning-induced plasticity steels through immersion and electrochemical polarization tests. The dual-phase steel exhibited the lowest resistance to pitting corrosion because of the high density of the inclusions present in it [4]. Michailidis et al. investigated the corrosion fatigue of galvanized and untreated high-strength steels through finite element modeling simulation to examine their in-situ stress fields and to elucidate their crack propagation mechanism [5]. Lu et al. also examined the corrosion behavior of high-strength 15Cr martensitic stainless steels under harsh conditions. They found that the corrosion of these steels was the most serious in acidic solutions. The corrosion rate in acidic solutions was found to be constant and was higher than that obtained in spent acid and formation water containing CO2. acidizing corrosion inhibitor displays a good matching ability with the high-strength 15Cr martensitic stainless steel in terms of decreasing the uniform corrosion rate, which changes mainly the anodic process of high-strength 15Cr martensitic stainless steel [6]. Wang et al. investigated the corrosion behavior of steels in neutral/acidic solutions containing NaCl by potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and scanning electron microscopy (SEM). They found that the pH and chloride concentration of the solutions had a significant influence on the pitting morphology of the steel samples. They observed a unique large pit morphology for the samples exposed to the solutions containing 0.05 mol/L NaCl [7].
Most of the studies mentioned thus far focused on the effect of the acidity, alkalinity, pH, and chloride concentration of corrosive media on the corrosion behavior of high-strength steels and ignored the effect marine water. The corrosive conditions used in these studies were different from the natural seawater environmental conditions. In addition, the experiments required long testing processes.
The splash and tidal zones of the marine environment offer the most severe corrosive conditions as they are prone to ebbs and high tides, which not only make them oxygen-rich but also increase the conductivity of sea water. Thus, marine corrosion of steel has been recognized as a critical problem in designing structures which are used in the splash and tidal zones. To overcome this problem, many marine steels have been developed. According to Song, the use of marine steels ensure a better corrosion protection of steel structures exposed to marine environments and reduces the cost of corrosion protection [8]. In this study, a new high-strength steel was developed and was named as P500. The steel was exposed to marine environments and was subjected to a cyclic corrosion test to measure its corrosion rate by the weight loss method. Pitting depth was also measured to determine the corrosion resistance of the steel.
In this study, the corrosion degradation mechanism of this newly developed steel was elucidated by carrying out an in situ corrosion test in a natural marine environment.
2. Experimental Method
Four different specimens, namely, SS400, A690, P500, and P600 were used. To evaluate the corrosion resistance of the marine steel, various tests were conducted. The exposure corrosion test was conducted by exposing the specimens for two years in three sea areas in Korea (Shiwa: west coast, Gwangyang: south coast, and Pohang: east coast). The specimens were exposed to the atmospheric, tidal, splash, and submerged zones of the marine environment. The specimens had a dimension of 150 mm × 80 mm × 10 mm. Figure 1 shows the test specimens subjected to the exposure corrosion test. They were connected electrically for simulating the steel pile exposed to the real environmental conditions.
Figure 2 shows the test specimens installed at each location for the exposure test. First, the marine growth attached to the surface of the specimens was removed mechanically. The specimens were then taken to the laboratory where they were cleaned ultrasonically and chemically according to KS D ISO 8407.
The specimens were immersed in a solution containing hydrochloric acid (HCl), di-antimony trioxide (Sb2O3), and stannic chloride pentahydrate (SnCl4) and were cleaned ultrasonically. The cleaning conditions are listed in Table 1. After the cleaning, the specimens were rinsed with ethanol for eliminating the grease and corrosion products present on their surface.
The pitting depth of the specimens was also measured to observe the pitting corrosion of the marine steel. After measuring the pitting depth, extreme value statistics were utilized to the calculate the life expectancy of the specimens.
3. Experimental Results
First, the extraneous materials (for example marine growth) present on the surface of the specimens were removed mechanically. Then, the underlying surface was cleaned chemically to remove the corrosion products formed on it. Most of the corrosion products were removed in this stage. Figure 3 shows the mechanically and chemically cleaned surfaces of SS400. After the fabrication and surface preparation, the specimens were weighed to the nearest 0.1 mg, exposed to the corrosive environment, cleaned of the corrosion products, and weighed again to determine the weight loss. The corrosion rates were calculated later.
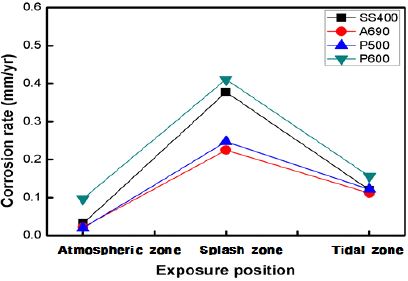
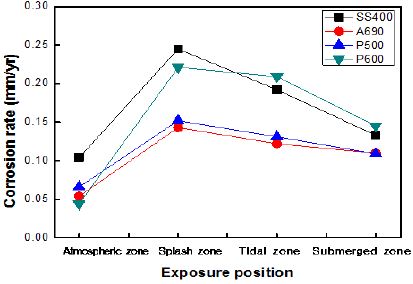
Figure 4 shows the corrosion rates of the specimens exposed to the marine environment in Shiwa, as calculated by the weight loss method. The corrosion rate in the splash zone was 2–10 times higher than that in the other zones. The corrosion rate was higher in the tidal zone than that in the atmospheric zone. The corrosion rates of SS400 and P600 were much higher than those of A690 and P500. P600 showed a high corrosion rate under every exposure condition. It has been reported that the corrosion in the tidal and splash zones is nearly the same. Under some conditions, the splash zone can induce a more severe corrosion than the tidal zone.
This implies that the influential factors in corrosion are varied in marine condition. In harsh weather, at points just above the waterline, the supply of chlorides increases vigorously because of the seawater splash, and the oxygen concentration also increases [9].
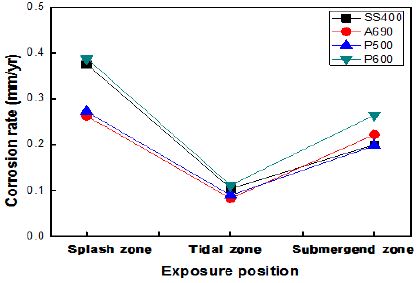
Figure 5 shows the corrosion rates of the specimens exposed to the marine environment in Gwangyang. The marine environment here consisted of three zones, namely, the splash, tidal, and submerged zones. The atmospheric zone was excluded because of ambiguous ebbs and high tides. The corrosion rate in the splash zone was 3–4 times higher than that in the other zones. The corrosion rate in the submerged zone was two times higher than that in the tidal zone. The corrosion rates of SS400 and P600 were much higher than those of A690 and P500. P600 showed a high corrosion rate under every exposure condition. The corrosion rates of the specimens taken from Gwangyang were lower than those of the specimens taken from Shiwa owing to the effect of ebbs and high tides.
The corrosion rates of the specimens exposed to the marine environment in Pohang, as calculated by the weight loss method are shown in Figure 6. Here, the marine environment divided into four zones, namely, the atmospheric, splash, tidal, and submerged zones. The corrosion rate in the splash zone was 2–4 times than that in the other zones. The corrosion rate in the atmospheric zone was lower than that in any other zone. The corrosion rates of SS400 and P600 were much higher than those of A690 and P500. P600 and SS400 showed 1.5–2 times higher corrosion rates under every exposure condition than the other specimens.
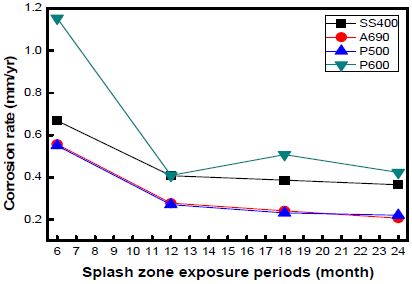
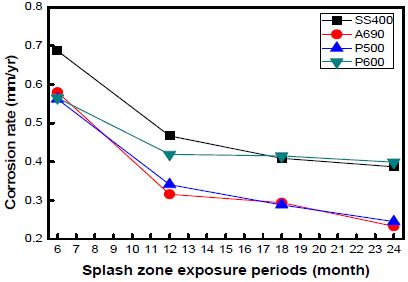
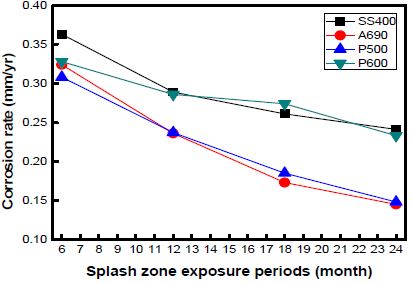
The corrosion rates of the specimens as a function of the exposure time at every location are shown in Figures 7, 8, and 9. The graph shows the variations in the corrosion rates of the specimens taken from every location as a function of the exposure time. The corrosion rates of SS400 and P600 were markedly higher than those of the other specimens at every location. This is attributed to the formation of chromium layers in these specimens.
The corrosion rate was far higher during the initial 12 months of immersion than that during the later 12 months. In the case of the specimens taken from Shiwa, the corrosion rate stabilized after an exposure time of 12 months. Many factors contribute to the decrease in the corrosion rate.
However, with an increase in the corrosion time, the corrosion rate under each condition gradually decreased and became constant.
The pitting depth showed a similar trend as shown by the corrosion rate. The P500, SS400, and P600 specimens taken from Gwangyang showed a pitting depth of 1.85, 2.70, and 2.61 mm, respectively. The P500, SS400, and A690 specimens taken from Pohang showed a pitting depth of 0.49, 1.95, and 0.95 mm, respectively. P500 was not susceptible to the pitting corrosion in the marine environments.
4. Conclusions
In this study, various corrosion tests were carried out to measure the corrosion rate and pitting resistance of the specimens. The following results were obtained.
- 1) The corrosion rate in the splash zone was much higher than that in any other zone.
- 2) P500 showed a high corrosion resistance in all the zones, except the splash zone.
- 3) P500 and A690 showed lower corrosion rates and pitting sensitivity after an exposure time of two years.
The exposure test revealed that P500 has lower corrosion rate than carbon steel. In addition, the pitting sensitivity of P500 was lower than that of the other specimens.
References
- D. A. Jones, Principle and Prevention of Corroaion, 2nd Edition, Prentice Hall, (1996).
-
R. Feng, J. Beck, M. Ziomek-Moroz, and , S. N, “Electrochemical corrosion of ultra-high strength carbon steel in alkaline brines containing hydrogen sulfide,”, Journal of Electrochemica Acta, 212(10), p998-1009, (2016).
[https://doi.org/10.1016/j.electacta.2016.07.070]

-
X. Guo, T. Shi, Z. Zhang, and B. Ma, “Stress corrosion cracking behavior of high strength casing steel in working fluids,”, Journal of Natural Gas Science and Engineering, 29, p134-140, (2015).
[https://doi.org/10.1016/j.jngse.2015.12.050]

- I. J. Park, S. M. Lee, M. Kang, S. Lee, and Y. K. Lee, “Pitting corrosion behavior in advanced high strength steels,”, Journal of Alloys and Compounds, 619(15), p205-210, (2014).
-
N. Michailidis, F. Stergioudi, G. Maliaris, and A. Tsouknidas, “Influence of galvanization on the corrosion fatigue performance of high-strength steel,”, Surface and Coatings Technology, 259(Part C), p456-464, (2014).
[https://doi.org/10.1016/j.surfcoat.2014.10.049]

-
X. H. Lu, F. X. Zhang, X. T. Yang, J. F. Xie, G. X. Zhao, and Y. Xue, “Corrosion performance of high strength 15Cr martensitic stainless steel in severe environments,”, Journal of Iron and Steel Research, International, 21(8), p774-780, (2014).
[https://doi.org/10.1016/S1006-706X(14)60140-0]

-
Y. Wang, G. Cheng, W. Wu, Q. Qiao, Y. Li, and X. Li, “Effect of pH and chloride on the micro-mechanism of pitting corrosion for high strength pipeline steel in aerated NaCl solutions,”, Applied Surface Science, 345(15), p746-756, (2015).
[https://doi.org/10.1016/j.apsusc.2015.05.053]

-
F. M. Song, “Predicting the Mechanisms and Crack Growth Rates of Pipelines Undergoing Stress Corrosion Cracking at High pH,”, Corrosion Science, 51(11), p2657-2658, (2009).
[https://doi.org/10.1016/j.corsci.2009.06.051]

-
J. A. Jeong, and C. K. Jin, “The experimental measurement on the throwing power of sacrificial anode cathodic protection for concrete piles in natural sea water,”, Advanced Materials Research, 1125, p350-354, (2015).
[https://doi.org/10.4028/www.scientific.net/amr.1125.350]