Effects of various densities and velocities on gaseous hydrocarbon fuel on near nozzle flow field under different laminar coflow diffusion flames

Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
An experimental study on the flow characteristics under various laminar coflow diffusion flames was conducted with a particular focus on the buoyancy force exerted from gaseous hydrocarbon fuels. Methane (CH4), ethylene (C2H4), and n-butane (C4H10) were used as the fuels. A coflow burner and the Schlieren imaging technique were used to observe the flow field of each fuel near the nozzle exit as well as the flow characteristics in the flames. The results show that a vortex with a density heavier than air appeared in n-butane near the nozzle exit with a strong negative buoyancy on the fuel steam. As the Reynolds number increased through the control of the fuel velocity of the n-butane flame, the vortices were greater and the vortex tips were moved up from the nozzle exit. In addition, the heated nozzle affected the flow fields of the fuel steam near the nozzle exit.
Keywords:
Laminar diffusion flame, n-Butane, Recirculation zone, Reynolds number, Buoyancy1. Introduction
Toxic emissions are responsible for the severe problems caused to the human health and environment, including vegetation and climate. Many studies related to the control of emissions and the flow structure of a combustion flame have been conducted [1][2], which are also an interesting subject in the field of marine engineering.
Moreover, laminar coflow diffusion flames have been investigated in studies on emissions and the hydrodynamic structure of various hydrocarbon fuels, while taking into consideration the different densities of hydrocarbon [3]. The dynamic structure of a buoyant jet on a diffusion flame, heavy fuel density, and volumetric expansion were affected on the vortex structures [4][5]. In the case of fuels with heavier densities than air, a recirculation zone appears near the nozzle exit [3][6].
Various studies on coflow/counterflow diffusion flames have also been carried out. Such investigations have focused closely on a flow field analysis using computational fluid dynamics and experiments clarifying the characteristics of the flame zone [7].
According to [8], the buoyancy of a diffusion flame can affect a burned gas region. Diffusion flames can be inspected between the fuel and oxidized zones. The flow fields of a hydrodynamic structure in a laminar diffusion flame have also been studied [9]. In addition, varying Reynolds numbers affecting the flame bulge region and a complex vortex effect near the nozzle exit have been investigated [10]. However, studies on the buoyancy effect on the recirculation zone in a flame for different fuels have been very limited. The purpose of the present study is to investigate the flow characteristics near the nozzle exit as well as flame structures with different fuel densities through a coflow with air. In addition, the effects of a heated nozzle on the flow field near the nozzle exit are also investigated.
In particular, to investigate the hydrodynamic structure of a normal coflow diffusion flame with different fuel types and various fuel densities, the Schlieren imaging technique was utilized to observe the flow field in different diffusion flames. In addition, we focused on the recirculation zones in different laminar flow flames.
2. Experimental setup
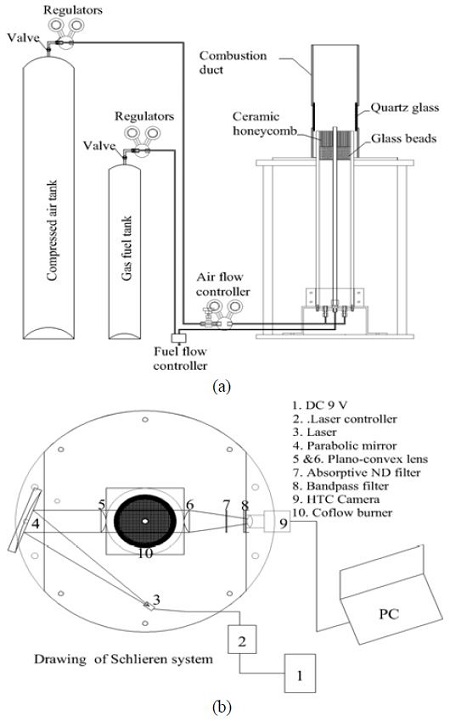
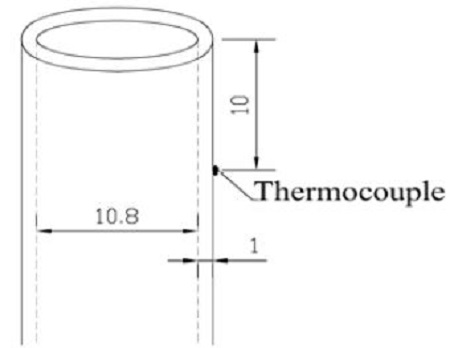
Figure 1 shows schematics of the coflow burner setup (a) and Schlieren system (b) used in the experiment. The fuel nozzle used consisted of a stainless steel tube with an inner diameter of 10.8 mm and a thickness of 1.0 mm, and the length of the nozzle was 655 mm to allow the flow of the fuel to be located within the laminar regime. A coflow tube made out of a transparent acrylic cylinder with an inner diameter of 100 mm, 10 mm thickness, and 640 mm length was also utilized.
The coflow air was passed through small glass beads and a ceramic honeycomb to make a uniform air flow.
Methane, ethylene, and n-butane (purity > 99.5%) were selected as the gaseous fuels. The fuel velocities were 4.45 cm/s for methane and 2.21 cm/s for ethylene. In the case of n-butane, the fuel velocities varied from 0.54 cm/s to 1.52 cm/s. The air velocities were 3.08 to 7.19 cm/s. Mass flow controllers were used to control the flow rate of hydrocarbon fuel and air.
For the flame structure and observations of the flow field in the flame near the nozzle exit, the Schlieren system was adopted. This system consists of an HVR-2300CB camera, a single mode fiber-pigtailed laser with a laser controller, a 9 V DC power supply, a parabolic mirror, and other supportive equipment.
A bright light source is directed toward the mirror, and the refracted light passes through the first plano-convex lens and the test region. The parallel rays pass through the second plano- convex lens, an absorptive neutral density filter, and a band-pass filter, and then through the light toward the camera.
3. Results and discussion
3.1 Flame structure
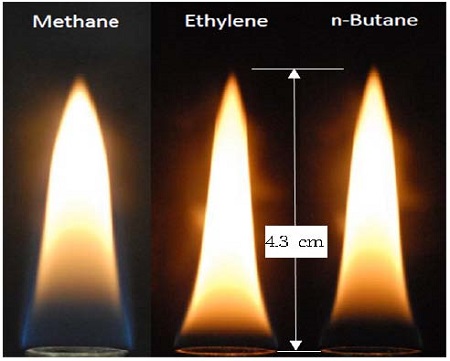
Figure 2 shows images of a direct flame for different fuels. The velocities for methane, ethylene, and n-butane were 4.43, 2.23, and 1.09 cm/s, respectively. The different velocities were selected to maintain the same flame height, as shown in Figure 2. Coflow air was supplied at a velocity of 6.16 cm/s. The velocity of air keeps the flame in a steady state.
It can be seen from Figure 2 that the height for the three flames is nearly the same at Hf = 4.3 cm. For circular-port flames, the flame height depends on the initial volumetric flow rate and the fuel specifications [11]. The following expression can be used to estimate the flame length of a circular- port burner [11]:
| (1) |
QF is the initial volumetric flow rate, S is the molar stoichiometric oxidizer fuel-ratio, and T∞ and TF are the ambient and fuel stream temperatures. The molar stoichiometric oxidizer fuel-ratio depends on the chemical composition of both the nozzle fluid stream and the surrounding fluid. The molar stoichiometric air fuel ratio for a generic hydrocarbon CxHy can be expressed as
| (2) |
where Xo2 is the mole fraction of oxygen in the air. Different brightness levels and flame zone structures are caused by different fuel characteristics [12]. In particular, the difference in brightness is due to soot. Methane has less soot compared with ethylene and n-butane [3]. For the methane flame structure, the blue zone was higher than in the ethylene and n-butane flames. The luminous zone of the ethylene flame was longer than that of the methane and n-butane flames owing to the fuel-specific soot generated [13].
3.2 Effect of heated nozzle to flow field near nozzle exit
This study investigated the effects of a heated nozzle on the flow field near the nozzle exit in coflow diffusion flames. The nozzle absorbs heat from the flame and transfers the heat to the fuel steam. Therefore, the velocity of fuel is increased at the nozzle exit from the heated nozzle.
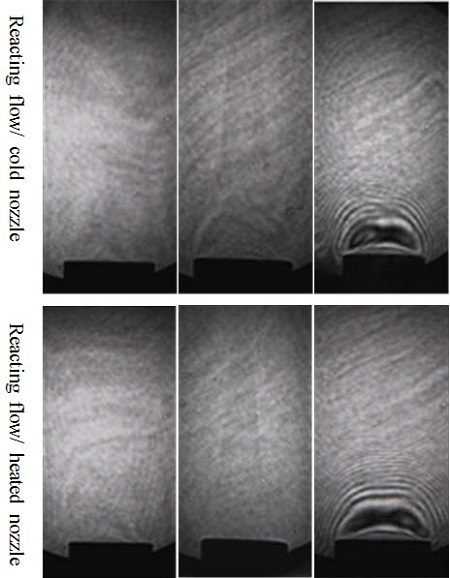
Y. Xiong et al[3] conducted an experiment using three different nozzle conditions: (1) a cold flow and cold nozzle, (2) a cold flow and heated nozzle, and (3) a reacting flow and cold nozzle.
Their results indicate that, in the case of a cold flow and cold nozzle, the fuel densities of propane and n-butane were greater than the air and buoyancy being directed vertically downwards and thus the fuel steam rapidly lost axial momentum and fell from the nozzle exit. In the case of a cold flow and heated nozzle, immediately after blowing off the flame, the flow fields showed a large-scale vortex near the nozzle exit owing to the heated nozzle. For the reacting flow and cold nozzle, the recirculation zones near the nozzle were smaller than for the cold flow and heated nozzle case.
For this experiment, one more case was derived, i.e., a reacting flow and heated nozzle using a methane, ethylene, and n-butane flame, as shown in Figure 3. The reacting flow and heated nozzle with an n-butane flame showed that the recirculation zone was greater than for the reacting flow and cold nozzle owing to an increased fuel velocity by reacting with the heated nozzle. The nozzle temperature was 156 °C as measured at 10 mm below the nozzle exit with n-butane as the fuel.
For the methane and ethylene flames, the fuel steam rapidly moved axially upward toward the centerline owing to the buoyancy and the densities of these fuels being lighter than air. Near the nozzle exit, the fuels steam did not move within the pocket zone. Therefore, methane and ethylene flames did not appear in the recirculation zone near the nozzle exit.
3.3 Effects of nozzle material properties and fuel type on heated nozzle
A laminar coflow diffusion flame is very sensitive to the fuel type, fuel density, temperature, pressure, and material properties [12][14][15]. Gulder et al. [16] studied the effects of fuel nozzle material properties on the soot formation and temperature field in a coflow diffusion flame. The results showed that the temperature of the nozzles differed when different fuel types were burned (propylene and ethylene), when different nozzle materials (aluminum, steel, or glass) were used, and for different measurement locations of 3 mm and 16.5 mm below the nozzle exit. In the case of a steel tube with ethylene fuel, the highest nozzle temperature of 124 °C was measured at 3 mm below the nozzle exit.
In this study, methane, ethylene, and n-butane were each burned using a stainless steel tube. The nozzle temperature was measured at 10 mm below the nozzle exit. The results show that the maximum temperatures of the burner for a methane flame, ethylene flame, and n-butane flame are 160 °C, 205 °C, and 156 °C, respectively. The nozzle temperature of the stainless steel tube (205 °C) was higher than that of the steel tube (125 °C) when using the same ethylene fuel. In the case of ethylene fuel with the stainless steel nozzle tube, the temperature was higher than for the other cases (aluminum, steel, and glass materials, while burning methane, propylene, and n-butane). The ethylene flame was affected by heated nozzle more than the methane and n-butane flames. The material properties of the nozzle caused heat to transfer from the flame to the nozzle tube at different temperatures.
3.4 Effects of Reynolds number on recirculation zone in n-Butane
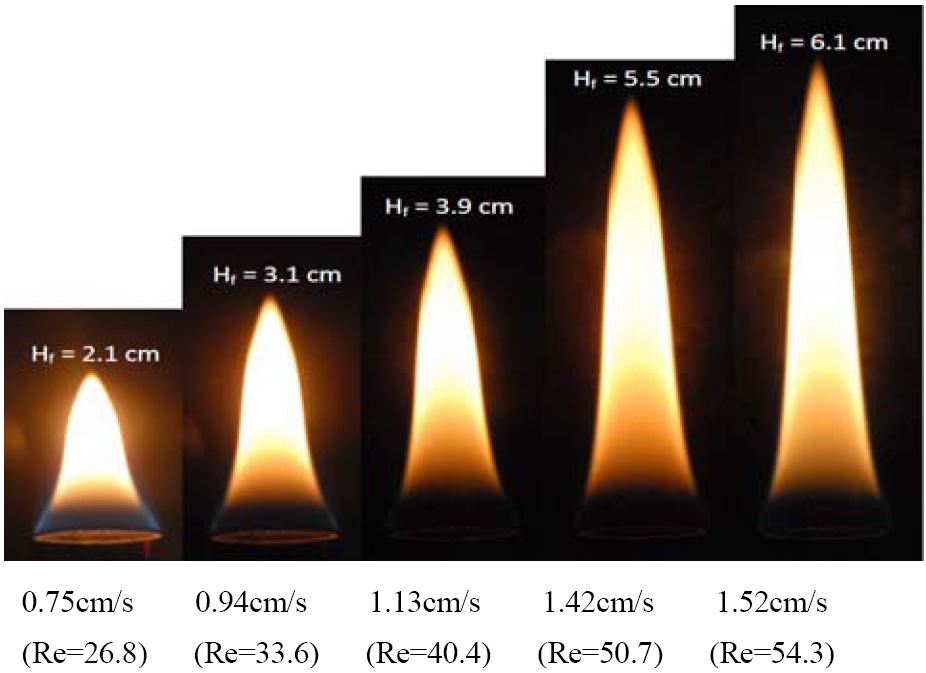
Figure 5 shows images of direct flames for various velocities of n-butane. The length of the laminar diffusion flame is dependent on the initial conditions. The flame length depends on the stoichiometric fuel mass fraction and volumetric flow of hydrocarbon [11]. For the circular-port flames, the flame lengths were increased owing to the increased volumetric flow rate of the fuel.
Figure 6 shows the Schlieren images for various velocities of n-butane. The velocity of n-butane required to create various Reynolds numbers for the recirculation zone at the flow field of the coflow diffusion flame was varied. The Reynolds number is defined as
| (3) |
where D represents the internal tube diameter, V represents the velocity, ρ represents the density, υ represents the kinematic viscosity, and μ represents the dynamic viscosity.
In Equation (3), if the velocity of the fuel increases the Reynolds number will be increased. The Reynolds numbers for each flame are 26.8, 33.6, 40.4, 50.7, and 54.3 with various velocities of 0.75, 0.94, 1.13, 1.42, and 1.52 cm/s, respectively. Fuel was supplied through the inner nozzle and the coflow air velocity was 6.16 cm/s. The results show the effect of the increased fuel velocity of n-butane on the recirculation zone near the nozzle exit.
In the case of 0.75 cm/s of the fuel velocity, the small vortices were positioned near the nozzle exit. Because the fuel velocity of n-butane increased up to 0.94, 1.13, 1.42, and 1.52 cm/s, the vortexes were greater than that at a low velocity. As the fuel velocity increased, the centerline was moved up from the nozzle exit and downstream of the vortex backward from the centerline. Therefore, the recirculation zone became higher and wider, increasing the fuel velocity.
4. Conclusion
The flow characteristics in methane, ethylene, and n-butane coflow diffusion flames, while accounting for the buoyancy effect, were experimentally investigated. In particular, the flow field near the nozzle exit was investigated. The results can be summarized as follows:
- 1. According to an investigation into the flame structure, the flame brightness and flame zone are characterized by the properties of the fuel. The length of the flame from a circular-port is dependent on the volumetric flow rate and fuel specifications.
- 2. The density of n-butane is heavier than air, and a vortex appears near the nozzle exit with strong negative buoyancy on the gas stream.
- 3. A heated nozzle can affect the flow fields of a fuel stream near the nozzle exit. In particular, for a reacting flow and heated nozzle the n-butane flame shows a greater recirculation zone than in the case of a reacting flow and cold nozzle owing to the increased fuel velocity when reacting with the heated nozzle.
- 4. Because the Reynolds number increases through the control of the velocity, the vortices in an n-butane flame are greater and the vortex tip in the flame is moved up from the nozzle exit.
References
-
J. H. Choi, “Experimental study on characteristics of synergistic effect of fuel mixing on number density and size of soot in ethylene-base counterflow diffusion flames by laser techniques”, Journal of the Korean Society of Marine Engineering, 33(3), p378-386, (2009).
[https://doi.org/10.5916/jkosme.2009.33.3.378]

-
J. H. Choi, and S. K. Park, “A numerical study on soot formation in ethylene diffusion flame under 1g and 0g”, Journal of the Korean Society of Marine Engineering, 37(8), p807-815, (2013).
[https://doi.org/10.5916/jkosme.2013.37.8.807]

-
Y. Xiong, M. S. Cha, and S. H. Chung, “Fuel density effect on near nozzle flow field in small laminar coflow diffusion flames”, Proceedings of the Combustion Institute, 35(1), p873-880, (2015).
[https://doi.org/10.1016/j.proci.2014.06.025]

-
V. R. Katta, and W. M. Roquemore, “Role of inner and outer structures in transitional jet diffusion flame”, Combust and Flame, 92, p274-282, (1993).
[https://doi.org/10.1016/0010-2180(93)90039-6]

-
R. W. Davis, E. F. Moore, W. M. Roquemore, L. D. Chen, V. Vilimpoc, and L. P. Goss, “Preliminary results of a numerical-experiment study of the dynamic structure of a buoyant jet diffusion flame”, Combust and Flame, 83, p263-270, (1991).
[https://doi.org/10.1016/0010-2180(91)90074-L]

- J. Buckmaster, and N. Peters, “The infinite candle and Its stability-A paradigm for flickering diffusion flames”, Symposium (International) on Combustion, 21(1), p1829-1836, (1988).
-
N. A. Eaves, A. Veshkini, C. Riese, Q. Zhang, S. B. Dworkin, and M. J. Thomson, “A numerical study of high pressure, laminar, sooting, ethylene-air coflow diffusion flames”, Combust and Flame, 159, p3179-3190, (2012).
[https://doi.org/10.1016/j.combustflame.2012.03.017]

- H. Gotoda, Y. Asano, K. H. Chuah, and G. Kushida, “Nonlinear analysis on Dynamic behavior of Buoyancy-Induced Flame Oscillation Under Swirling Flow”, International Journal of Heat and Mass Transfer, 52(23-34), p5423-5432, (2009).
-
D. Trees, T. M. Brown, K. Seshadri, M. D. Smooke, G. Balakrishnan, R. W. Pitz, V. Giovangigli, and S. P. Nandula, “The structure of nonpremixed hydrogen- air flames”, Combustion Science and Technology, 104(4-6), p427-439, (1995).
[https://doi.org/10.1080/00102209508907731]

-
F. G. Roper, “The prediction of laminar jet diffusion flame sizes: Part I. theoretical model”, Combust and Flame, 29, p219-234, (1977).
[https://doi.org/10.1016/0010-2180(77)90112-2]

- S. R. Turnes, An Introduction to Combustion Concepts and Applications, McGraw-Hill, USA, (1976).
-
M. R. J. Charest, C. P. T. Groth, and O¨ .L. Gu, ¨ lder, “A numerical study on the effects of pressure and gravity in laminar ethylene diffusion flames”, Combust and Flame, 158, p1933-1945, (2011).
[https://doi.org/10.1016/j.combustflame.2011.02.022]

-
M. A. Mikofski, T. C. williams, C. R. Shaddix, and L. G. Blevins, “Flame height measurement of laminar inverse diffusion flames”, Combust and Flame, 146, p63-72, (2006).
[https://doi.org/10.1016/j.combustflame.2006.04.006]

-
Ö. L. Gülder, “Soot formation in laminar diffusion flames at elevated temperatures”, Combust and Flame, 88, p74-82, (1992).
[https://doi.org/10.1016/0010-2180(92)90008-D]

-
Ö. L. Gülder, “Influence of sulfur dioxide on soot formation in diffusion flames”, Combusta and Flame, 92, p410-418, (1993).
[https://doi.org/10.1016/0010-2180(93)90152-S]

-
Ö. L. Gülder, K. A. Thomson, and D. R. Snelling, “Effect of fuel nozzle material properties on soot formation and temperature field in coflow laminar diffusion flames”, Combust and Flame, 144, p426-433, (2006).
[https://doi.org/10.1016/j.combustflame.2005.10.003]