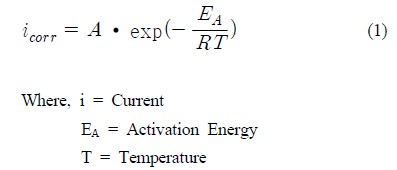The effect of temperature and relative humidity on concrete slab specimens with impressed current cathodic protection system
Impressed current cathodic protection (ICCP) system is one of the most promising corrosion protection methods. The Effect of ICCP system can be changed at diverse conditions. Particularly, temperature and relative humidity plays a crucial role in CP (Cathodic Protection) effect. Thus, in this study, the influence of temperature and relative humidity on concrete specimens was investigated. Specimens were concrete slab type with a base of 400mm × 400mm and height of 70mm. To enhance the effect of CP system, seawater was used as an electrolyte. Used anode for ICCP system was mixed metal oxide (MMO) titanium. Test factors were natural potential, CP potential, CP current, and 4-hour depolarization potential. From this study, it could be confirm that CP potential and current were highly influenced by temperature and relative humidity.
Keywords:
Cathodic protection, Concrete structure, ICCP, Temperature, Relative humidity1. Introduction
Reinforced concrete is a versatile, economical and successful construction material [1][2]. Generally, concrete has a high alkaline environment since it has a lot of microscopic pores that contain high concentration of soluble calcium, sodium, and potassium oxides. These form hydroxides, which are high alkaline(pH 12-13). Due to this fact, concrete structures normally consider non-corrosive condition [3]. However, if there are any detrimental elements, such as de-icing salt and carbon dioxide, the concrete structure deteriorates quickly than life expectancy [4][5]. To protect corrosion damage, CP has been used for decades and it becomes one of the proven technologies, especially in harbor structures. CP is an electrical method for corrosion protection that can be applied to metal exposed to conductive circumstance. It has been known as an optimal way to protect corrosion [6]. However, as if it is a proven technique, it is difficult to forecast exact lifespan of structures. This is because the behavior of CP varies depending on the exposed environment of structures. Particularly, temperature and RH play an important role in the behavior of CP. In case of temperature and RH, not many studies, related to reinforced concrete structures, have been conducted [7]-[11]. Relationship between temperature and CP effect could be revealed by Arrhenius Equation (1). According to Arrhenius equation, when temperature is increased, the amount of current also rises [12]. However, in case of RH, it was difficult to prove the exact relation between RH and CP behavior. All electrochemical processes take place in pore solution in concrete. The moisture content thus plays a crucial role in chemical reactions in concrete. Furthermore, in real concrete structure, there are many complex variables such as resistivity, and the concentration of chloride and/or carbon dioxide. Therefore, to understand the effect of temperature and RH, some experiments considering real natural environment should be conducted.
2. Experimental Method
2.1 Specimen
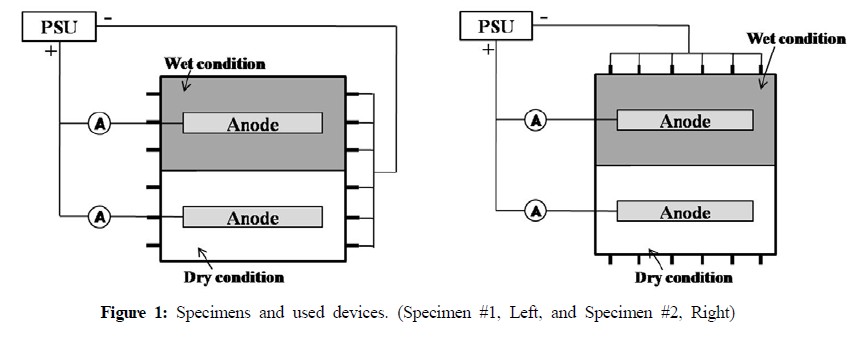
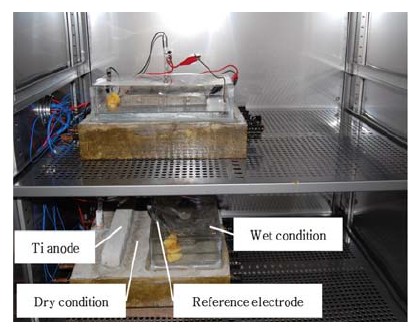
Figure 1 and 2 show specimens and experimental devices. As shown in Figure 1 and 2, Specimens were slap type reinforced concrete structures with a base of 400mm × 400mm and height of 70mm. The reinforced steel was deformed rebar manufactured by means of a typical carbon steel (KS D16), and the interval between rebar was 60mm. Mixed metal oxide (MMO) titanium (ribbon type) was used as anode for ICCP to supply CP current. After casting, the specimen was cured in laboratory at a room temperature (20±3℃) for 30 days. The design of CP is shown in Figure 1. To verify seawater effect, the specimen was divided into 2 sections, i.e. immersed zone (Wet condition, seawater), and non-immersed one (Dry condition, air). Moreover, in this study, the specimen of two different types was used. Rebar in specimen were arranged not only vertically in concrete (#1 specimen), but also horizontally in concrete (#2 specimen). Current flows from rectifier to wet and dry areas. The setting potential of wet areas for ICCP was -760mV vs. SSCE (-850mV vs. CSE), which is the CP criterion for carbon steel measured by CSE according to NACE SP 0290 [13]. Ti-ribbon anode was applied to each portion to supply current. Rectifier to supply current was CR-1212 Power Supply Unit (CORREL Technology), which can monitor the potential and current, and 4-hour depolarization potential. Silver/silver chloride electrode (SSCE) was used as a reference electrode to measure potential. Furthermore, Temp & Humidity chamber (HANBAEK SCIENTIFIC CORPORATION) was used to sustain temperature and RH (Figure 3).
2.2 Experimental Procedures
Table 1 gives experimental circumstance. To verify the effect of temperature, temperature of 20, 30 and 40℃ at RH of 85% was selected. In addition, RH of 75, 85, and 95% at temperature of 30℃ was selected to understand the effect of RH. Each condition was sustained for about one week until potential was stabilized. Measured factors were CP Potential, CP current, and 4 hour depolarization potential. Especially, 100mV depolarization potential criterion was adopted according to the NACE CP criterion [14].
3. Experimental Results
3.1 Temperature Variation
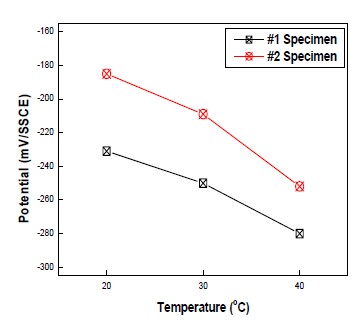
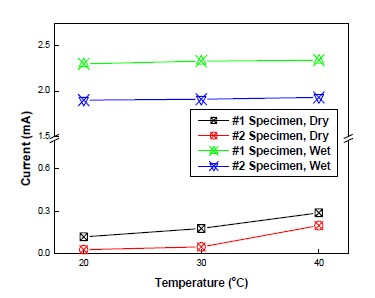
Figure 4 shows the CP potential variation of dry areas at different temperature and same humidity. At identical temperature, Potential of #1 and #2 specimen was sustained at -760mV vs. SSCE in wet areas. However, in dry area, potential of #1 and #2 at temperature of 20℃ was different each other, depending on the inside condition of specimen (#1 specimen was -231mV vs SSCE, and #2 specimen was -185mV vs SSCE). In this study, the potential of #1 specimen had a tendency to be lower than that of #2 specimen. In addition, an increase in temperature leaded to a decrease in potential of specimen, which was same inclination in both #1 and #2 specimens. In addition, as explained earlier, potential changed according to Arrhenius equation. Potential change from 30℃ to 4 0℃ was about 1.5 times higher than that from 20℃ to 30℃
Figure 5 shows a correlation between CP current and temperature. As shown in Figure 5, the current of wet areas sustained its values regardless of temperature variation. This is because although temperature in concrete was changed, resistivity in concrete didn’t change in wet areas. Thus, this phenomenon prevented CP current from increasing. In contrast, the current of dry areas varied due to temperature variation. The higher temperature was, the higher value current shown. Furthermore, CP current in dry areas increased a lot at from 30℃ to 40℃.
3.2 Humidity Variation
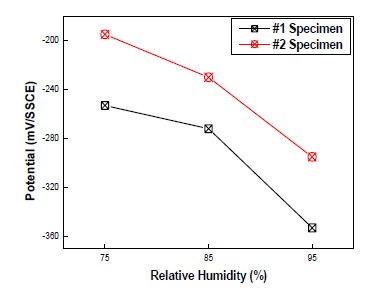
Figure 6 shows the effect of RH on potential at RH of 75%, 85%, and 95% and temperature of 20℃. As mentioned earlier, RH has a significant influence on a protection reaction in concrete. In this case, humidity was closely related to potential variation. When RH was increased, potential dropped significantly. The reason of potential drop was a increase in inner humidity, which leaded to the decrease in resistivity. At RH of 95%, potential drop was about 2.2 times bigger than potential drop at from RH of 75% to 85%. In addition, compared to the temperature variation at identical RH, potential drop at humidity changes was much bigger. Furthermore, the decrease in potential, depending on a rise in RH, was higher in #1 specimen than #2 specimen. This was because the arrangement between rebar and anode was paralleled, which leaded to focusing CP current on rebar.
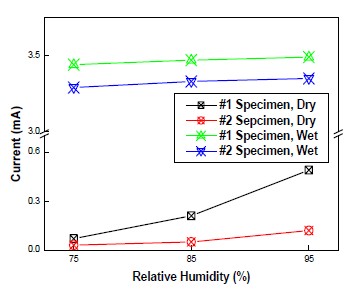
Figure 7 shows the current variation at different RH. The current of wet area did not change and sustained its own values because these regions were already filled with water, and RH could not play an important role in current and potential changes. Conversely, in dry areas, current values were determined by RH. Compared to the result of temperature variation, when RH of dry areas was increased, the bigger current supply could be observed. Considering temperature and RH, RH had some crucial impact on CP potential and current. This was thought that RH leaded to decreasing resistivity in concrete, which contributed to more flexible state that had a great possibility of chemical reactions. By contrast, the rise in temperature leaded to increased resistivity in concrete specimen, which reduced the effect of temperature on a protection behavior.
3.3 4-Hour Depolarization Measurement
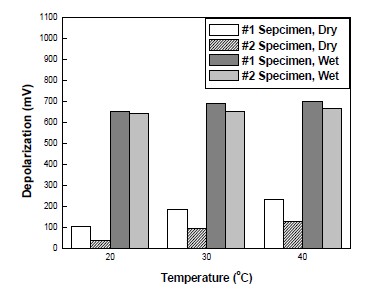
Depolarization tests have been regularly carried out by disconnecting the anodes from rebar for 4-hours or 24-hours. 100mV depolarization criterion was selected to prove the effectiveness of CP. In this study, while the potential of wet areas was secured at -760mV vs SSCE, that of dry areas was different, depending on temperature and/or RH. Therefore, by comparing the 4-hour depolarization of dry areas, the effectiveness of CP at various temperatures and RH could be identified. Figure 8 and 9 show the result of 4-hour depolarization measurements. As shown in Figure 8, the depolarization potential of wet areas showed huge values ranged from 690mV to 640mV. In spite of temperature variation at identical RH, depolarization potential was similar in wet areas. In contrast, there were some differences between each dry condition. As temperature was increased, depolarization potential was also increased. #1 specimen showed higher depolarization potential changes than #2 specimen at identical temperature.
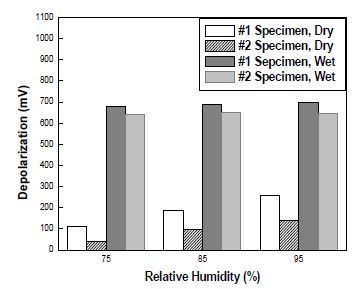
As shown in Figure 9, the depolarization potential was changed when RH was changed. As RH was increase, the depolarization potential in dry areas was also risen, which was a same tendency to previous potential potential variation results. In wet areas, However, RH rarely affected specimen because concrete pores were already filled with water. Considering all factors influencing concrete specimen, temperature and RH play a crucial role in the efficacy of CP. Therefore, it needs to consider temperature and RH in order for the effective design of a CP system in concrete structures.
4. Conclusion
From the results, regarding the influence of ICCP system on slab type concrete specimens at different temperatures and RH, following results have been obtained:
1) As temperature was increased, CP potential was dropped. In addition, the potential changes at different temperature tended to change linearly. In other words, a potential change from temperature of 30℃ to 40℃ was about 1.5 times higher than a potential change from temperature of 20℃ to 30℃.
2) RH also showed a similar inclination to temperature variation results. The higher RH was, the lower values potential showed. Moreover, RH had more influences on the potential change of concrete specimens than temperature.
3) Depolarization potential varied at different temperature or RH. Therefore, it was essential to take these factors into account for effective design of CP system in reinforced concrete structures.
References
- J. P Broomfiend, Corrosion of Steel in Concrete 2nd Edition, Taylor & Francis, (2007).
-
M. Kouril, P. Bovak, and M. Carcasses, Threshold chloride concentration for stainless steels activation in concrete pore solutions, Cement and Concrete Research, 40, p431-436, (2010).
[https://doi.org/10.1016/j.cemconres.2009.11.005]

- M. Raupach, B. Elasense, and R. Polder, Corrosion of Reinforcement in Concrete, European Federation of Corrosion Publications, (2007).
-
T. S. Nguyen, S. Lorente, and M Carcasses, Effect of environment temperature on the chloride diffusion though CEM-I and CEM-V mortars : an experimental study, Construction and Building Materials, (2009), 23, p795-803.
[https://doi.org/10.1016/j.conbuildmat.2008.03.004]

- M. H. Im, Cavitation characteristics on impeller materials of centrifugal pump for ship in sea water and fresh water, corrosion science and technology, 10, p218, (2011).
-
T. D Rincon, Y. H Lopez, A. D Valle-Moreno, A. A Torres-Acosta, F. B Pablo Moreno, P. O Salinas, J. R Montero, Environmental influence on point anodes performance in reinforced concrete, Concrete and Building Material, 22, p494-503, (2008).
[https://doi.org/10.1016/j.conbuildmat.2006.11.014]

- J. G. Nam, and W. H. Hartt, “Effects of relative humidity and temperature on chloride diffusion and time-to-corrosion of reinforcing steel in concrete, National Association of Corrosion Engineers International, 05257, p1-18, (2005).
- A. Raharinaivo, I. Pepenar, and G. Grimaldi, Influence of temperature on the potential of reinforcing steel in concrete, under cathodic protection, corrosion of reinforcement in concrete construction, Royal Society of Chemistry, 183, p369-377, (1996).
- M. Rasheeduzzafar, and G. Ali, Effect of temperature on cathodic protection criterion for reinforced concrete structures, American Concrete Institute Special Publications, 139, p21-40, (1993).
-
F. Hunkeler, The resistivity of pore water solution - a decisive parameter of rebar corrosion and repair methods, Construction and Building Materials, 10, p381-389, (1996).
[https://doi.org/10.1016/0950-0618(95)00029-1]

-
M. Saleem, M. Shameem, S. E. Hussain, and M. Maslehuddin, Effect of moisture, chloride and sulphate contamination on the electrical resistivity of portland cement concrete, construction and building materials, 10, p209-214, (1996).
[https://doi.org/10.1016/0950-0618(95)00078-X]

- F. Pruckner, Corrosion and Protection of Reinforcement in Concrete Measurements and Interpretation, Ph.D Dissertation, Department of National Sciences and Mathematics, University of Vienna, Austria, (2001).
- National Association of Corrosion Engineers International, “Impressed Current Cathodic Protection of Reinforcing Steel in Atmospherically Exposed Concrete Structures”, U.S., Standard SP0290, Jun, 16), (2007.
- National Association of Corrosion Engineers International, “Control of External Corrosion on Underground or Submerged Metallic Piping Systems”, U.S., Standard SP0169, Mar, 15), (2007.