The feasibility of ammonia as marine fuel and its application on a medium-size LPG/ammonia carrier
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The study investigated the feasibility of using ammonia as a marine fuel and its application on a medium-size LPG/ammonia carrier. The study included a comprehensive literature review of existing research on ammonia as a marine fuel and further research on industrial developments in the field to gain insights into the practical challenges and opportunities associated with using ammonia as a marine fuel. The study found that ammonia has several advantages as a marine fuel, including its high energy density, low emissions profile, and potential for production from renewable sources. However, there are also significant technical, economic, and safety challenges associated with using ammonia, particularly in the design and operation of marine engines and fuel systems, particularly due to ammonia’s combustion characteristics. The study also demonstrated that the application of ammonia on a medium size LPG/Ammonia carrier is feasible but requires investment and technical expertise. The findings of this study have important implications for the shipping industry's efforts for decarbonization and suggest that ammonia could play a significant role in a carbon-free maritime future. In conclusion, the study provides a comprehensive analysis of the feasibility of ammonia as a marine fuel and highlights its potential application on a medium size LPG/Ammonia carrier.
Keywords:
Alternative fuels, Ammonia, Decarbonization, Sustainability, Carbon free maritime1. Introduction
Shipping is a major contributor to global greenhouse gas (GHG) emissions and its emissions have a significant impact on the climate. The sector's emissions are expected to continue to grow in the coming decades unless steps are taken to reduce them. According to the International Maritime Organization (IMO), a United Nations agency that is responsible for regulating shipping and the prevention of pollution from ships, shipping was responsible for about 2.76% of global GHG emissions in 2012 which was increased to 2.89% in 2018, and these emissions are expected to increase by 90~130% by 2050, depending on future economic and energy trends [1].
The IMO has set several targets and initiatives to reduce the shipping industry's GHG emissions, including, the adoption of the Initial IMO Strategy on Reduction of GHG Emissions from Ships in 2018, which sets out a vision for the sector to reduce its total annual GHG emissions by at least 50% by 2050, compared to 2008 levels [2].
There are several initiatives underway to address the shipping industry's emissions, including the development of alternative fuels such as biofuels, hydrogen, and the use of more efficient engines and fuel systems as well as ammonia which is one of the most promising options for the future of shipping. The IMO has also set targets for reducing the sector's CO₂ emissions and is working to develop a global strategy for reducing GHG emissions from ships.
Several initiatives are underway to address the shipping industry's emissions, including the development of alternative fuels such as biofuels, hydrogen, and the use of more efficient engines and fuel systems as well as ammonia. The IMO has also set targets for reducing the sector's CO₂ emissions and is working to develop a global strategy for reducing GHG emissions from ships.
Ammonia has received increasing interest as a potential marine fuel in recent years due to its potential to reduce GHG emissions from shipping. Unlike fossil fuels, ammonia does not produce carbon dioxide when burned, making it an attractive alternative for decarbonizing the shipping industry. Additionally, ammonia is widely available and can be produced using renewable energy sources, further reducing its environmental impact. However, there are also challenges associated with the use of ammonia as a marine fuel, including the need for specialized storage and handling equipment, and the potential for emissions of nitrogen oxides during combustion. Further research and development are needed to determine the feasibility and viability of ammonia as a marine fuel on a larger scale.
Overall, the IMO's efforts aim to reduce the shipping industry's GHG emissions cost-effectively and practically, while also considering the sector's need for a level playing field and the need to support economic growth.
2. Emissions and regulations
Governments at all levels have put in place several laws and regulations to decrease pollution and the release of harmful substances and to advance sustainable growth.
The Kyoto Protocol, which was signed at the United Nations (UN) Climate Change Conference in 1997 agreed to lower emissions of gases such as carbon dioxide, methane, and sulfur hexafluoride by 5% by 2012 in comparison to 1990 levels [3][4].
The Paris Agreement aims to keep the global temperature increase below 2 degrees Celsius, with a target of 1.5 degrees Celsius, through the reduction of GHG emissions [5].
The Green Deal initiative of the European Union (EU) aims to decrease GHG emissions by at least 55% by 2030 compared to 1990 levels and become carbon neutral by 2050 [6].
The United Nations Earth Summits for environmental protection and sustainable development were held in 1992, 2002, and 2012 [9], and also the United Nations has been organizing climate conferences since 1995 the result of these activities was the adoption of the Millennium Development Goals in 2000 and the 2030 Sustainable Development Goals in 2017 [7].
The International Convention for the Prevention of Pollution from Ships (MARPOL) Annex VI, implemented in 1997, sets limits on key pollutants in ship exhaust including Sulphur oxides (SOx) and Nitrogen oxides (NOx) as well as prohibits the release of ozone-depleting substances (ODS) [8].
In addition, the Energy Efficiency Design Index (EEDI) is a key technical measure that aims to encourage the use of more energy-efficient (less polluting) equipment and engines in new ships which was a critical step taken by IMO. The EEDI is calculated using a formula based on the ship's technical design parameters and is expressed in grams of CO₂ per ship's capacity mile, with a lower EEDI indicating a more energy-efficient ship design [9].
In addition to EEDI, starting from January 1, 2023, it will be mandatory for all ships to calculate their Energy Efficiency Existing Ship Index (EEXI) to measure their energy efficiency and to begin collecting data for reporting their annual operational Carbon Intensity Indicator (CII) and CII rating. The mandatory EEXI and CII regulations were implemented as part of the Initial IMO Strategy for Reducing GHG Emissions from Ships, which was established in 2018 and the adoption of EEXI and CII falls under the short-term objectives, which include the aim to decrease the carbon emissions of international shipping by 40% by the year 2030, as compared to the levels in 2008 [10].
Another issue that IMO is working on development of guidelines for the assessment of the GHG Lifecycle. The Intersessional Working Group will provide an update on its progress in creating draft guidelines for assessing the entire lifecycle of GHG emissions, including the production and usage of alternative marine fuels [11]. These guidelines, known as Lifecycle GHG assessment (LCA) guidelines, will provide a way to calculate emissions from the "well-to-wake" process, including emissions from the production to storage, and usage phases.
3. Overview of alternative fuels and their comparison
The conversation about reducing carbon emissions in the shipping industry has gained momentum in recent years. The increasing research on different types of alternative marine fuels highlights the challenges in evaluating marine fuels and the lack of a consistent approach to assess alternative marine fuels holistically.
Some of the most promising alternative marine fuels are liquefied natural gas (LNG), liquefied petroleum gas (LPG), hydrogen, methanol, biofuels, and ammonia which are studied, and a comparison is done based on several fuel characteristics such as flammability, which can affect safety during handling, storage, and use, toxicity, that can pose risks to human health and the environment, the energy density as a crucial factor in determining the practicality of a fuel for marine applications and lastly, the maturity of technology which is based on the availability in maritime industry for application. Overall, the choice of alternative marine fuel will depend on a careful consideration of these and other factors, as well as the specific needs and priorities of the maritime industry.
3.1 Liquefied natural gas
LNG in some respects is considered a cleaner fossil fuel as it produces less SOx, NOx, and PM in comparison to heavy fuel oil and distillate fuels, however, methane (CH₄) is a primary component of natural gas and is a powerful GHG and has a radiative force that is 30 times stronger than CO₂ over a period of 100 years [12]. This means that the potential benefits of natural gas are being questioned due to the leakage of methane (methane slip) during production, transportation, and combustion, especially at low speed and low load operations of natural gas compression engines [12]. It is crucial to gain a better understanding of the GHG emissions from LNG related activities to achieve national and international climate goals and global climate objectives.
3.2 Liquefied petroleum gas
At normal temperature and pressure, LPG is in gaseous form, however, when it is under pressure or cooled, it changes to a liquid form which is the handling principle onboard LPG carriers. LPG is a suitable and environmentally friendly fuel option and due to its unique features and cost-effectiveness, it can be considered as a good choice for certain vessel types [13].
While LPG has a higher energy content than some other alternative fuels such as alcohols, which makes it suitable for use in modern dual-fuel engines, however, it has not been widely adopted as compared to LNG due to its lower ability to reduce emissions and the different safety concerns it poses [14].
3.3 Methanol
Methanol (CH₃OH or MeOH), also known as wood alcohol, is a clear, colorless liquid that is widely used as a solvent, fuel, and chemical intermediate [15]. As a marine fuel, methanol has gained attention for its potential to reduce emissions, increase energy efficiency and decrease the dependence on fossil fuels.
Methanol offers several advantages as a marine fuel and does not have the same storage and handling requirements as LNG or heavy fuel oil (HFO) as it is a liquid at normal temperatures, does not need to be stored at low temperatures or require specialized materials for tanks and pipes which on the other hand require more space onboard than LNG or HFO for the same voyage distance [16]. When methanol is burnt, it produces carbon dioxide and water, and the emissions from burning fossil-derived methanol in ship engines are much lower than those of heavy fuel oil.
3.4 Hydrogen
Hydrogen is the most prevalent element in the universe, and it has the greatest energy storage capacity per unit weight of any energy carrier [17]. One of the mostly discussed alternative marine fuel is Hydrogen which is gaining attention as a potential solution to the shipping industry's need to reduce emissions and transition to cleaner energy sources.
One way to use hydrogen as a fuel is by keeping it in a liquid state, which can then be utilized in modified gas turbines or internal combustion engines (ICEs) while another technology that utilizes hydrogen is fuel cells (FC) as a promising alternative to traditional marine fuels, which use catalytic reactions on the ship to convert traditional hydrocarbon fuels into hydrogen to generate electricity where the primary byproduct of FC when utilizing hydrogen is water only, and making it a highly environmentally friendly option [18].
The storage of hydrogen for maritime applications poses unique challenges compared to stationary or automotive applications, hence the storage of hydrogen for ships is more difficult than in these other scenarios [19].
3.5 Biofuels
The use of biofuels in marine applications can potentially decrease the output of pollutants such as SOx, NOx, and Particular Matter (PM), resulting in a better air quality worldwide and specifically in harbors in comparison to the usage of marine diesel or heavy fuel oil [20]. Biofuels have been shown to decrease overall carbon emissions because of the absorption of carbon from the atmosphere during the growth of the biomass and may become more important in the future if produced from more renewables [21]. Another advantage that makes biofuels an attractive option for marine use is that they can be utilized as a ready-to-use drop-in fuel with minimal modifications required for existing ship engines and infrastructure such as bunkering vessels [22]. There are various types of biofuels that can be used for marine applications, and some of those are straight vegetable oils (SVO), biodiesel (FAME), hydrotreated vegetable oil (HVO), biomethane, and biogas.
3.6 Ammonia
Ammonia (NH₃), a chemical compound made up of one nitrogen atom and three hydrogen atoms, is used as an inhibitor in certain enzymatic reactions and is toxic to the nervous system which can be both artificially created in industrial settings and naturally produced through bacterial actions and the decay of organic material [23]. When it is combusted, it produces only water and nitrogen, making it a near-zero-emission fuel [24].
The use of ammonia as a marine fuel has the potential to provide significant economic and environmental benefits such as having the potential to reduce ship fuel costs and improve energy efficiency along with its huge potential to significantly reduce GHG emissions that shipping industry has been fighting for.
There are three commonly used methods for producing ammonia: using natural gas which is called steam methane reforming (SMR), coal gasification, or water electrolysis combined with the Haber-Bosch (H-B) process and most of the ammonia produced currently is from fossil fuels and primarily made through the combination of SMR and the H-B process [25].
While it is possible to produce green ammonia using electrolysis and renewable energy, this method is more energy-intensive and less efficient, resulting in higher costs and additionally, the equipment used in electrolysis is costly, which further increases the expense of green ammonia production [25].
More research and development are required to reduce the cost of green ammonia production, but the advantages of utilizing ammonia as an alternative fuel make it a potential alternative for the future. This study examined the benefits and drawbacks of using ammonia as an alternative fuel, and it is obvious that more study is needed to make it a viable choice.
3.7 Comparison of alternative fuels
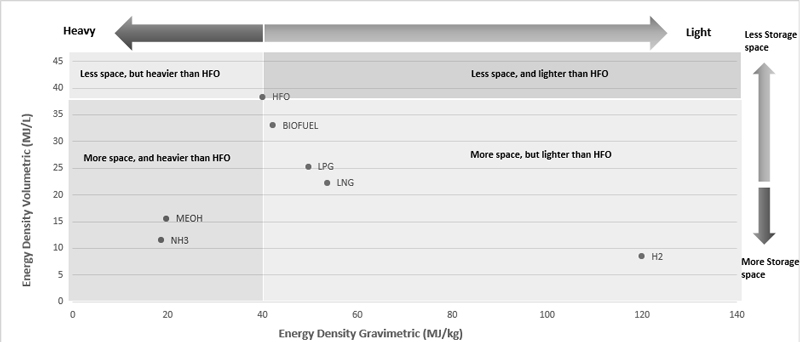
Fuels with high energy density (kJ/kg) and specific energy (kJ/m3) are preferred ones since they require a smaller storage tank and have a lighter weight. Overall system efficiency and energy consumption or losses are normally incorporated in determining the fuel mass and volume for a more precise assessment of the mass and volume for fuels onboard the ship. The gravimetric energy density and volumetric energy density of alternative fuels studied are shown in Figure 1 and HFO is used as base fuel for comparison and according to data indicated that only the mass and volume requirements are shown and if the storage tank capacities are also considered, then there will be a significant change for certain fuels like those require cryogenic/refrigerated or pressurized fuel containment systems such as Hydrogen (H₂) which has gravimetric densities lower than 10 MJ/kg for LH₂ including containment systems, compared to 119.93 MJ/kg for the fuel only while for the LNG gravimetric and volumetric densities of ~22.2 MJ/kg and ~13 MJ/L including storage systems, respectively, compared to ~53.6 MJ/kg and ~21 MJ/L for the fuel only [26].
Another major concern is safety which is needed to be assessed, particularly due to the toxicity and flammability, and for that reason the use of alternative fuels in maritime is strictly regulated and more under development such as for Ammonia. To have a better understanding on this, an overview of critical properties connected with alternative fuels is assessed and shown in Table 1.
The development of rules for the marine application of alternative fuels is crucial as it ensures equivalent safety standards and reduces the risk levels involved. By implementing equivalent safety principles in the design and operation of vessels, the use of alternative fuels can be made more reliable and sustainable. The potential benefits of NH₃ as alternative fuel, such as reducing emissions and improving efficiency, can only be fully realized if the necessary precautions are taken to mitigate the increased risk levels for the toxicity that can only come with new technologies. Therefore, the development of comprehensive rules must be a priority to ensure a safer and greener future for the maritime industry.
4. Ammonia as marine fuel
4.1 Current state of research on ammonia as marine fuel
Numerous research and development projects are underway, working to establish standards and protocols for the safe and efficient use of NH₃ as a fuel. As these standards are developed and refined, the conditions and applications for the use of NH₃ in the maritime industry will become clearer.
Engine designers are currently researching both combustion concepts, but due to the fuel slip issues inherent in the Otto cycle process, the difficulty in initiating NH₃ combustion, and its slow burn characteristics, the Diesel cycle is thought to be the most suitable combustion concept: in fact, it is being selected by MAN-ES for their ME-LGIA engine concept [29]. While a significant progress has done, the main concern remains to be the fuel combustion as the fuel injectors have received extensive development and testing, with a focus on the materials employed to support not just efficient combustion but also lifecycle maintenance [30].
Wartsila, another major engine manufacturer is researching both combustion concepts, including the use of NH₃ and methane in Otto (or Diesel) cycles to minimize CO₂ emissions [29]. The company’s 25 medium-speed 4-stroke engine which can already run on diesel, LNG, or liquid carbon-neutral biofuels, was recently introduced and it can be simply upgraded to run on future carbon-free fuels as they become available such as NH₃ [31].
WinGD, one of the key engine manufacturers for low pressure LNG Dual Fuel (DF) engines run on Otto cycle, and Hyundai Heavy Industries are partnering to create the first WinGD two-stroke engine capable of using NH₃ as fuel by 2025 [32].
In addition to developments happen progressively on technology side, there are also significant progress has been made for regulatory sides for adoption of NH₃. The Maritime Safety Committee’s (MSC) 105th session, which convened from April 20 to 29, agreed that the IMO should include "Development of nonmandatory standards for the safety of ships utilizing ammonia as fuel" to its work schedule which will be carried out by the Sub-Committee on Cargo and Container Carriage (CCC), with a completion date of 2023 [33].
4.2 Challenges of using ammonia as marine fuel
Despite its potential benefits, the use of ammonia as a marine fuel still faces a few problems and barriers that must be overcome before it can be extensively utilized and adopted in a wide range.
Presently the main issue with NH₃ is production from renewables as green NH₃ due to WtW GHG emission issues, however current production methods make it more expensive than the conventional NH₃. The limited availability of infrastructure for bunkering with ammonia makes it difficult to implement this fuel on a large scale. This is especially true in remote and coastal areas, where it is difficult to build the infrastructure required to handle this fuel.
As NH₃ is a hazardous material, and there are significant risks associated with its handling and storage those include fires, explosions, and toxic gas releases, which can pose a threat to the safety of ship crews and the surrounding environment especially if this will be associated with also lack of experience and appropriate training as all will create additional safety risks, as it is essential to have trained personnel who understand the unique properties and hazards associated with this fuel.
The use of NH₃ as a marine fuel is not yet regulated in the same way that traditional fuels, however dissections at IMO level are ongoing as NH₃ is considered a toxic product and is currently not permitted for use under IGC code, which will require amendment in the long term.
4.3 Propulsion and power generation technologies
Because of the properties of NH₃, its use presents some challenges as it has a substantially lower heating value compared to other hydrocarbons. NH₃ also has a low flammability due to its narrow equivalency ratio (0.63 to 1.4) and high auto ignition temperature. The adiabatic flame temperature of NH₃ is 1800 ⁰C, which is lower than that of H₂ (2110 ⁰C), CH₄ (1950 ⁰C), and C₃H₈ (2000 ⁰C) and as a result, radiation heat transmission is reduced, which is critical during combustion and heat transfer [34].
Fuel Cell and combustion-based systems are the two major approaches which can be used to utilize NH₃. In a simple expression, FC systems employ a chemical process to transform NH₃ energy into electrical power, whereas combustion-based systems use the heat created by burning NH₃ to power a mechanical device such as an engine or turbine.
There are various FC types are available and they are classified based on their working parameters as well as electrolyte material and six of the major systems [35], namely, Solid Oxide Fuel Cell (SOFC), Proton Exchange Membrane (PEM), Alkaline FC (AFC), Direct Methanol FC, Phosphoric Acid FC (PAFC) and Molten Carbonate Fuel Cell (MCFC) are available in the market.
NH₃ powered FC have also been extensively researched for stationary power production and as transportation power sources. Although most cells developed to date use Hydrogen (H₂) as fuel, and it is acknowledged that storing H₂ for such purposes is still difficult and expensive [36].
Investigations using NH₃, which undergoes thermal cracking within a high temperature fuel cell to produce H₂ and N₂ at the anode, have revealed that the maximum amount of work that can be obtained from NH₃ is 0.33 MJ/mol, whereas if the work is calculated in terms of the H₂ that can be obtained from NH₃ cracking, the maximum value is 0.22 MJ/mol, a value that is similar to other conventional fuels [36][37]. As the NH₃ is cracked by the anode within the cell, there is no need for a front-line NH₃ reformer, and so NH₃ may be utilized indirectly to create the needed H₂ for energy generation in fuel cells [38].
NH₃, as previously mentioned, may also be utilized as a fuel for ICEs and due to its high octane (110-130) [39], like that of other alternative fuels such as CH₃, H₂ and CH₄, can enhance combustion qualities and lessen undesired effects like as knocking [40]. Additionally, studies shows that NH₃-fueled engines feature lower power losses, no corrosion, and use less lubrication than traditional fuels.
On the other hand, while NH₃ in ICEs is stated as viable for NH₃/fossil fuel or other alternative fuel combinations, pure NH₃ combustion in engines has yet to be shown a reliable method due to various challenges, hence changes such as introducing better injection design and improvements that allow the engine to function at higher temperatures as well as pressures than normal values might be required. These changes eventually may reduce emissions and improve the combustion kinetics and dynamics [41]. To overcome such challenges in NH₃ combustion, in 2020 using the engine at high compression ratios was proposed in a study as a solution, however, was not successful [42]. In the context of the NH₃ engine, it was observed that low reactivity of NH₃, along with a high compression ratio, led significant pressure peak [45]. To address this issue and optimize engine performance, a diesel pilot fuel was introduced which enabled reduction in compression ratio to normal levels.
Another power generating device can run on NH₃ is gas turbine which is relatively old in industrial applications. When dealing with the usage of NH₃ fuel in gas turbines, one of the most significant factors to address is NOx emissions as they are likely to be higher compared other fuels due to the fuel NOx mechanism, since NOx is mostly created by the thermal process in hydrocarbon flames and as a result, numerous researchers have concentrated their efforts on this topic, assessing the impact of various combustion parameters on NOx generation such as using different fuel mixes and equivalency ratios.
A variety of ways using NH₃ as a flexible fuel in gas turbines have recently been studied, with most of them discovering that emissions are the principal restriction of such technology [43] and a series of articles summarizing the major problems for the development of a reliable technology were provided [44] those are: a) lower flame temperatures and slower NH₃ kinetics; b) unstable combustion; c) reliable NH₃ vaporization to enhance efficiency; and d) molecule pre-cracking necessary to boost flame speed and burning rates.
5. Feasibility of ammonia on a medium size LPG/ammonia carrier
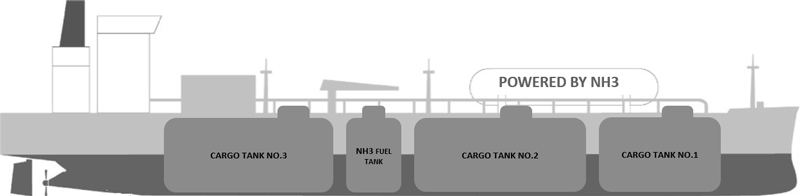
To assess the feasibility of NH₃ as a marine fuel, a case study of a medium-sized LPG- NH₃ carrier is conducted as shown in Figure 2 and Table 2. The selection of a medium-sized vessel is based on its ability to access a vast majority of ammonia ports worldwide and its potential to utilize the already available NH₃ cargo onboard as fuel. Additionally, medium-sized vessels are more versatile and adaptable to changing market conditions and can be more easily deployed on a variety of routes and trades. Moreover, choosing an LPG-NH₃ carrier as the pilot project can mitigate some of the challenges related to its toxicity and specific material requirements. This study aims to provide insights into the practical implementation of NH₃ as a marine fuel, highlighting its potential benefits and limitations.
5.1 Methodology of case study
There are structured and systematic approaches used for risk assessment and decision-making and in this case Structured What-If Technique (SWIFT) method is used to assess and evaluate the potential risks and uncertainties. SWIFT is a systematic and structured approach that involves identifying hypothetical scenarios by asking "what-if" questions. Here is the outline of steps involved in SWIFT method applied:
- • 1st step – Definition of scope and objective
- • 2nd step – Scenarios identification
- • 3rd step – Analyzing scenarios and consequences
- • 4th step – Assess the risks
- • 5th step – Identification of contingencies
- • 6th step – Review, revise and document the results
5.2 Onboard systems associated with ammonia fuel
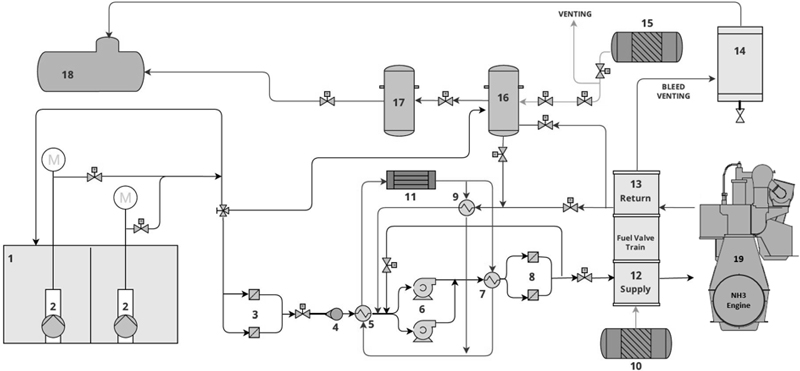
The NH₃ fuel supply to the engine is supplied via the fuel supply system (FSS) during dual-fuel operation as shown in Figure 3 and Table 3 along with all system components studied and evaluated.
5.3 Machinery and Fuel Preparation Spaces
In the typical arrangement for gas carriers where the cargo is used as fuel, there are two approaches for machinery space concepts, namely “Gas Safe” machinery space or “Non-hazardous” machinery space and ESD Machinery space.
For the spaces where the likelihood of fuel leakage may be higher, such as fuel-preparation rooms, additional safety measures and operational safeguards will be required. The fire and explosion hazards for NH₃ fuel must also be evaluated, but the toxicity risk will require suitable type of gas detector and subsequent alert levels which must be identified during the detailed HAZID study. For this study Gas Safe machinery space concept is used and a simplified HAZID result of which is mentioned under 5.3 is prepared based on this approach.
5.4 Risk assessment and HAZID
The purpose of the risk assessment and HAZID study is to identify the potential major hazards relative to the operational configuration of a proposed NH₃-fueled vessel at an early stage of concept development. This analysis helps in reviewing the effectiveness of selected safety measures and, where required, expand the safety measures to achieve tolerable levels of residual risk. The study seeks to:
- • Identify hazards arising from NH₃ as a marine fuel.
- • Evaluate the potential consequences of these hazards and assess their probability of occurrence.
- • Develop practical safeguards and risk mitigation measures
- • Provide clear and actionable recommendations for mitigating hazards.
- • Ensure that the use of NH₃ as a marine fuel meets the safety standards stipulated in the IMO IGC code.
- • Establish a robust framework for future safety-assessment studies.
To evaluate the importance of each hazard based on its frequency and severity, a risk matrix as shown in Table 4 is prepared. The risk levels are assigned based on the intersection of the severity level and frequency level. For example, a hazard with a Severity Level of 5 (Catastrophic) and a Frequency Level of 5 (Almost Certain) would be considered an "Extremely High" hazard with a residual risk (RR) factor of 25. Conversely, a hazard with a Severity Level of 1 (Minimal) and a Frequency Level of 1 (Rare) would be considered a "Low" hazard.
The project is only a conceptual design, but approach is based on present dual fuel systems used for LPG and Methanol carriers and their similarities along with the additional safety concerns identified under HAZID worksheet which is conducted separately and not included in this study due to its length.
During the HAZID worksheet development, 4 'extremely high' and 11 'high' risk scenarios were identified, which will require mitigation during the design phase and the summary of risk ranking along with the HAZID nodes those are developed and selected based on Table 3 are shown in the Table 5.
6. Conclusion
This study aimed to investigate the feasibility of using NH₃ as a marine fuel and its application on a medium-sized LPG/NH₃ carrier. The study's findings indicate that using NH₃ as a marine fuel is technically viable and can provide considerable environmental benefits over standard fossil fuels. Yet, certain challenges must be overcome for it to be widely adopted.
One of the main concerns is the building of NH₃-compatible engines. Because of its narrow flammability range and high autoignition temperature, NH₃ is difficult to ignite and burn in a standard engine. This causes technical challenges in engine design and operation, such as the demand for specialized fuel injection systems, optimizing combustion efficiency, and significant safety problems.
The construction of infrastructure for the storage, delivery, and handling of NH₃ as a marine fuel is one of the major issues. NH₃ is a toxic and corrosive material that must be handled with special attention. This infrastructure will need major investment and coordination from a variety of stakeholders, including shipbuilders, fuel producers, and regulatory bodies.
Additionally, the use of NH₃ as a marine fuel will require specific regulations, safety precautions, and personnel training to ensure the safety of crew and the environment for which IMO has been working and still in the development phase only.
In conclusion, this study has highlighted the potential of NH₃ as a marine fuel, with its multiple advantages over other alternative fuels. However, the adoption of NH₃ as a marine fuel will require ongoing research and development efforts to address technical challenges and ensure the safety and sustainability of its use.
Acknowledgments
This study was a part of a project entitled ‘Development of guidance for leakage prevention and mitigation of consequences in hydrogen ships’ funded by the Ministry of Oceans and Fisheries, Korea.
Author Contributions
Conceptualization, M. Akturk; Methodology, M. Akturk; Formal Analysis, M. Akturk; Investigation, M. Akturk; Resources, M. Akturk; Writing-Original Draft Preparation, M. Akturk ; Writing-Review & Editing, J. K. Seo; Visualization, M. Akturk; Supervision, J. K. Seo; Funding Acquisition, J. K. Seo.
References
- IMO (2020), Fourth Greenhouse Gas Study 2020, https://www.imo.org/en/OurWork/Environent/Pages/Fourth-IMO-Greenhouse-Gas-Study2020.aspx, Accessed June 1, 2023.
- IMO (2023), IMO’s work to cut GHG emissions from ships, https://www.imo.org/en/MediaCentre/HotTopics/Pages/Cutting-GHG-emissions.aspx, , Accessed June 1, 2023.
- United Nations Climate Change (2019), What is the Kyoto Protocol, UNFCCC, https://unfccc.int/kyoto_protocol, , Accessed June 1, 2023.
-
C. Breidenich, D. Magraw, A. Rowley, and J. Rubin, “The Kyoto protocol to the United Nations framework convention on climate change,” The American Journal of International Law, vol. 92, no. 2, p. 315, 1998.
[https://doi.org/10.2307/2998044]

-
L. E. Erickson and G. Brase, Reducing Greenhouse Gas Emissions and Improving Air Quality: Two Interrelated Global Challenges, New York, USA: CRC Press, 2019.
[https://doi.org/10.1201/9781351116589]

- European Commission. (2020), 2030 Climate Target Plan, https://climate.ec.europa.eu/eu-action/european-green-deal/2030-climate-target-plan_en, , Accessed June 26, 2023.
- United Nations (1992), United Nations Conference on Environment and Development, Rio de Janeiro, Brazil, June 3-14, 1992.
- IMO (2023), Prevention of Air Pollution from Ships, https://www.imo.org/en/about/Conventions/Pages/International-Convention-for-the-Prevention-of-Pollution-from-Ships-(MARPOL).aspx, , Accessed June 26, 2023.
- IMO (2023), Energy Efficiency Measures, https://www.imo.org/en/OurWork/Environment/Pages/Technical-and-Operational-Measures.aspx, Accessed June 1, 2023.
- IMO (2022), Rules on ship carbon intensity and rating system enter into force, https://imopublicsite.azurewebsites.net/en/MediaCentre/PressBriefings/pages/CII-and-EEXI-entry-into-force.aspx, Accessed February 18, 2023.
- IMO (2022), Media information – MEPC 78 preview, https://www.imo.org/en/MediaCentre/IMOMediaAccreditation/Pages/MEPC-78-preview-.aspx, , Accessed February 18, 2023.
-
B. Manouchehrinia, Z. Dong, and T. A. Gulliver, “Well-to-Propeller environmental assessment of natural gas as a marine transportation fuel in British Columbia,” Energy Reports, vol. 6, pp. 802-812, 2020.
[https://doi.org/10.1016/j.egyr.2020.03.016]

-
S. J. Yeo, J. Kim, and W. J. Lee, “Potential economic and environmental advantages of liquid petroleum gas as a marine fuel through analysis of registered ships in South Korea,” Journal of Cleaner Production, vol. 330, p. 129955, 2022.
[https://doi.org/10.1016/j.jclepro.2021.129955]

- ABS (2019), Setting the Course to Low Carbon Shipping - Pathways to Sustainable Shipping - Outlook II (low res), https://absinfo.eagle.org/acton/media/16130/setting-the-course-to-low-carbon-shipping-pathways-to-sustainable-shipping-outlook-ii-low, , Accessed February 18, 2023.
- PubChem (2019). Methanol. [online] Nih.gov. Available: https://pubchem.ncbi.nlm.nih.gov/compound/887, .
- J. Probst (2022), Methanol as an alternative fuel for container vessels. DNV. [online] Available: https://www.dnv.com/expert-story/maritime-impact/methanol-as-an-alternative-fuel-for-container-vessels.html, , Accessed February 18, 2023.
-
M. F. Hordeski, Alternative Fuels—The Future of Hydrogen, 3rd edition, New York, USA: River Publishers, 2020.
[https://doi.org/10.1201/9781003151753]

-
L. Pomaska and M. Acciaro, “Bridging the maritime-hydrogen cost-gap: real options analysis of policy alternatives,” Transportation Research Part D: Transport and Environment, vol. 107, p. 103283, 2022.
[https://doi.org/10.1016/j.trd.2022.103283]

-
L. V. Hoecke, L. Laffineur, R. Campe, P. Perreault, S. W. Verbruggen, and S. Lenaerts, “Challenges in the use of hydrogen for maritime applications,” Energy & Environmental Science, vol. 14, no. 2, pp. 815-843, 2021.
[https://doi.org/10.1039/D0EE01545H]

-
M. D. Kass, Z. Abdullah, M. J. Biddy, C. Drennan, Z. Haq, T. Hawkins, S. Jones, J. Holliday, D. E. Longman, S. Menter, E. Newes, T. J. Theiss, T. Thompson, M. Wang, Understanding the Opportunities Of Biofuels For Marine Shipping, ORNL/TM-2018/1080, Energy and Transportation Science Division, Oak Ridge National Laboratory, 2018.
[https://doi.org/10.2172/1490575]

-
E. C. D. Tan, T. R. Hawkins, U. Lee, L. Tao, P. A. Meyer, M. Wang, and T. Thompson, “Biofuel options for marine applications: technoeconomic and life-cycle analyses,” Environmental Science & Technology, vol. 55, no. 11, pp. 7561-7570, 2021.
[https://doi.org/10.1021/acs.est.0c06141]

-
S. Li, E. C. D. Tan, A. Dutta, L. J. Snowden-Swan, M. R. Thorson, K. K. Ramasamy, A. W. Bartling, R. Brasington, M. D. Kass, G. G. Zaimes, and T. R. Hawkins, “Techno-economic analysis of sustainable biofuels for marine transportation,” Environmental Science & Technology, vol. 56, no. 23, pp. 17206-17214, 2022.
[https://doi.org/10.1021/acs.est.2c03960]

- PubChem (n.d.), Ammonia, [online] pubchem.ncbi.nlm.nih.gov, . Available: https://pubchem.ncbi.nlm.nih.gov/compound/222, .
-
J. Li, S. Lai, D. Chen, T. Wu, N. Kobayashi, L. Deng, and L. Huang, “A review on combustion characteristics of ammonia as a carbon-free fuel,” Frontiers in Energy Research, vol. 9, 2021.
[https://doi.org/10.3389/fenrg.2021.760356]

- Q. Zhu (2021), Challenges and opportunities of using clean ammonia as a fuel. International Centre for Sustainable Carbon( ICSC), Available: https://www.sustainable-carbon.org/blogs/challenges-and-opportunities-of-usingclean-ammonia-as-a-fuel, .
- SEA-LNG (2019), Comparison of Alternative Marine Fuels, https://sea-lng.org/reports/comparison-of-alternative-marine-fuels/, , Accessed June 26, 2023.
- Wikipedia Contributors (2019), Energy density. [online] Wikipedia. Available: https://en.wikipedia.org/wiki/Energy_density, .
- Neste (2020), Neste Renewable Diesel Handbook, Espoo, Finland: Neste Proprietary Publication.
- EMSA (2022), Potential of Ammonia as Fuel in Shipping by ABS, CE-DELFT and ARCSILEA. European Maritime Safety Agency, Lisboa, Portugal, [online] Available: www.emsa.europa.eu.
- MarineLink (2023), MAN ES: Moving Forward on Ammonia Engines, [online] Available: https://www.marinelink.com/news/man-es-moving-forward-ammonia-engines-502260, Accessed February 18, 2023.
- V. Denton (2022), Wärtsilä launches new 4-stroke engine that can run on ammonia, [online] F&L Asia, Available: https://www.fuelsandlubes.com/wartsila-launches-new-4-stroke-engine-that-can-run-on-ammonia/, , Accessed February 18, 2023.
- Hyundai Heavy Industries (HHI) – Ammonia Energy Association, [online] Available: https://www.ammoniaenergy.org/organization/hyundai-heavy-industries/, , Accessed February 18, 2023.
- U. Einemo (2022), IMO to develop guidelines for safe use of ammonia, [online] IBIA. Available: http://ibia.net/2022/05/04/imo-to-develop-guidelines-forsafe-use-of-ammonia/, Accessed February 18, 2023.
-
M. Aziz, A. T. Wijayanta, and A. B. D. Nandiyanto, Ammonia as effective hydrogen storage: a review on production, storage and utilization, Energies, vol. 13, no. 12, p. 3062, 2020.
[https://doi.org/10.3390/en13123062]

-
A. Afif, N. Radenahmad, Q. Cheok, S. Shams, J. H. Kim, and A. K. Azad, “Ammonia-fed fuel cells: a comprehensive review,” Renewable and Sustainable Energy Reviews, vol. 60, pp. 822-835, 2016.
[https://doi.org/10.1016/j.rser.2016.01.120]

-
A. Valera-Medina, H. Xiao, M. Owen-Jones, W. I. F. David, and P. J. Bowen, “Ammonia for power,” Progress in Energy and Combustion Science, vol. 69, pp. 63-102, 2018.
[https://doi.org/10.1016/j.pecs.2018.07.001]

-
E. Baniasadi and I. Dincer, “Energy and exergy analyses of a combined ammonia-fed solid oxide fuel cell system for vehicular applications,” International Journal of Hydrogen Energy, vol. 36, no. 17, pp. 11128-11136, 2011.
[https://doi.org/10.1016/j.ijhydene.2011.04.234]

-
R. Lan and S. Tao, “Direct ammonia alkaline anion-exchange membrane fuel cells,” Electrochemical and Solid- State Letters, vol. 13, no. 8, p. B83, 2010.
[https://doi.org/10.1149/1.3428469]

-
O. Herbinet, P. Bartocci, and A. G. Dana, “On the use of ammonia as a fuel – A perspective,” Fuel Communications, vol. 11, p. 100064, 2022.
[https://doi.org/10.1016/j.jfueco.2022.100064]

-
M. Koike, H. Miyagawa, T. Suzuoki, and K. Ogasawara, “Ammonia as a hydrogen energy carrier and its application to internal combustion engines,” Sustainable Vehicle Technologies, vol. 61-71, 2012.
[https://doi.org/10.1533/9780857094575.2.61]

-
J. S. Cardoso, V. Silva, R. C. Rocha, M. J. Hall, M. Costa, and D. Eusébio, “Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines,” Journal of Cleaner Production, vol. 296, p. 126562, 2021.
[https://doi.org/10.1016/j.jclepro.2021.126562]

-
P. Dimitriou and R. Javaid, A review of ammonia as a compression ignition engine fuel, International Journal of Hydrogen Energy, vol. 45, no. 11, pp. 7098-7118, 2020.
[https://doi.org/10.1016/j.ijhydene.2019.12.209]

-
A. Karabeyoglu, B. Evans, J. Stevens, B. Cantwell, and D. Micheletti, “Development of ammonia based fuels for environmentally friendly power generation,” 10th International Energy Conversion Engineering Conference, Atlanta, Georgia, 2012.
[https://doi.org/10.2514/6.2012-4055]

- A. Karabeyoglu and B. Evans, Fuel Conditioning System for Ammonia-Fired Power Plants 9th Annual NH3 Fuel Association Conference, 2012.
-
C. Tornatore, L. Marchitto, P. Sabia, and M. D. Joannon, M. D., “Ammonia as green fuel in internal combustion engines: state-of-the-art and future perspectives,” Frontiers in Mechanical Engineering, vol. 8, 2022.
[https://doi.org/10.3389/fmech.2022.944201]