Engineering perspective of electrochlorination system for ballast water treatment using carbon dioxide
Copyright ⓒ The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Electrochlorination systems are widely used in ballast water treatment because they are more cost-effective than other disinfection techniques. Furthermore, the on-board generation of hypochlorous acid from electrochlorination systems has advantages in terms of footprint size and safety as there is no need to store highly concentrated hypochlorite solutions. Recently, several studies focused on the injection of carbon dioxide into an electrochlorination system to enhance disinfection efficacy. However, the treatment efficacy of injecting exhaust gas into an electrochlorination system remains understudied. In this study, the disinfection efficacy of Artemia sp. was investigated based on the holding time when carbon dioxide was injected into the electrochlorination system. Disinfection efficacy significantly improved as the pH decreased to 5.5. Additionally, the pH inside the ballast water tank adjusted based on ballast water flow capacity when the carbon dioxide from combustion engine was fully dissolved. Our results showed that the pH suitable for eliminating microorganisms was achieved using only the auxiliary engine without any acid additives. This study provides a promising conceptual design for ballast water treatment.
Keywords:
Ballast water treatment, Electrochlorination system, Disinfection efficacy, Exhaust gas, Carbon dioxide1. Introduction
Ballast water is transferred by vessels to ensure stability during voyages. Ballasting and deballasting operations are performed to offset changes in vessel displacements associated with unloading and loading, respectively. However, the various biological substances contained in ballast water can affect native species and cause ecological changes in the ocean [1].
To prevent invasive species from translocating via ballast water, ballast water should be treated and disinfected in accordance with the United States Coast Guard (USCG) and International Maritime Organization (IMO) regulations. In 2019, the IMO announced a list of approved ballast water treatment systems (BWTS) in accordance with the procedure. The 45 BWTS consists mainly of filtration, electrolysis, UV, ozone treatment, and chemical injection systems [2]-[3]. Among the BWTS, electrochlorination is preferred because it provides relatively low power consumption, cost, footprint area, and higher disinfection efficacy [3].
To enhance the removal of marine organisms, Cha et al. [4] examined the synergistic effects of carbon dioxide (CO2) injection on electrochlorination disinfection of Artemia franciscana. It is well known that injected CO2 can be converted into carbonic acid (H2CO3*) in water, leading to a decrease in the pH of water. At low pH, hypochlorous acid (HOCl) is formed more than the less powerful oxidative hypochlorite (OCl-). HOCl is 80-200 times stronger than OCl- in terms of pathogen disinfection [4]. In general, exhaust gas is a mixture of gases and contains mainly CO2, NOx, and SO2. Under these circumstances, CO2 generated from combustion engines can be a valuable source for the elimination of microorganisms (e.g., zooplankton and phytoplankton) because the exhaust gas can minimize energy consumption instead of the CO2 gas generation system. However, there are insufficient documents on the composition and treatment efficacy of exhaust gas in ballast water treatment to draw clear conclusions [5].
In this study, we focused on the influence of treatment efficacy by utilizing exhaust gas as a CO2 source and proposed a promising conceptual design for a new type of BWTS.
2. Experimental
2.1 Preparation of Test Water
The test water (100 L) was salinated (salinity > 32 PSU) using a mixture of dissolved mineral salts (Reef Salt, Aquaforest, Poland). The composition of the mixture was as follows: 19,000– 19,500 mg/L chlorine, 9,700–11,800 mg/L sodium, 1,300–1,360 mg/L magnesium, 810–990 mg/L sulfur, 410–430 mg/L calcium, and 360–380 mg/L potassium.
2.2 Microorganism Preparation and Enumeration
To meet the required test conditions stipulated in the IMO G8 guidelines, 105 individuals/m3 for organisms of size ≥ 50 ㎛ representing zooplankton, Artemia sp., which is relatively easy to culture and assay, was hatched from the dehydrated cysts. The temperature of the test water was maintained at 26oC for 2–3 days to provide oxygen during cyst development. Artemia sp. was enumerated using an optical microscope (Olympus SZ51). First, Artemia sp. was passed through 50 ㎛ a homemade mesh sieve. The volume of the concentrated sample was 50 mL and approximately 1mL was taken using an EppendorfTM micropipette. An appropriate amount of test water that did not contain living organisms was added so that light could pass through the Bogorov counting chamber well. During the visual inspection, the immobile Artemia sp. was checked by moving the needle to confirm whether the organism was alive.
2.3 Electrochlorination and CO2 Injection System
A laboratory-scale electrochlorination system was set up, and the same electrode as the HiBallastTM BWTS (IMO & USCG Type Approval Certificate) was used. The electrical current was controlled to adjust the total residual oxidant (TRO) concentration to 4 ppm using the same electrode. Afterward, CO2 (99.9%, KNG Co., Ltd.) was injected at a flow rate of 100–300 ml/min to lower the pH of the test water. For more detailed information, please refer to the schematic of the water treatment apparatus (see Figure S1).
2.4 Water Quality Parameter Measurements
The pH of the test water was measured using a pH meter (HM-40P; TOADKK, Japan). Dissolved oxygen (DO) concentration was measured using a DO meter (DO-31P; TOADKK, Japan), and the concentrations of chlorine (mg/L as Cl2) were determined using N,N-diethyl-p-phenylenediamine (DPD; Hach Company, USA) using a colorimetric method (See Figure S1)
3. Results and Discussion
Figure 1 shows the inactivation of Artemia sp. at different pH values in the presence of 4ppm TRO concentration. CO2 was used to adjust the pH of test water. When the pH was lowered from 8.2 to 5.5, the inactivation of Artemia sp. increased by approximately 3, 7, and 29 times on days 1, 2, and 3 of holding time, respectively. This agrees well with previous results that CO2 injection strengthens disinfection efficacy against microorganisms compared to the electrochlorination system alone [4][6]. According to earlier studies, the efficacy of electrochlorination relies heavily on HOCl concentration in water. Previous studies also explained that reduced pH increases the HOCl concentration in the solution, and the disinfection efficacy of HOCl is much higher than that of ClO- (considering that the pKa of HOCl is 7.53) [4][6]. Meanwhile, estimates showed that the disinfection efficacy greatly increased as the decomposition reaction of foreign substances accelerated and biological toxins increased on day 3.
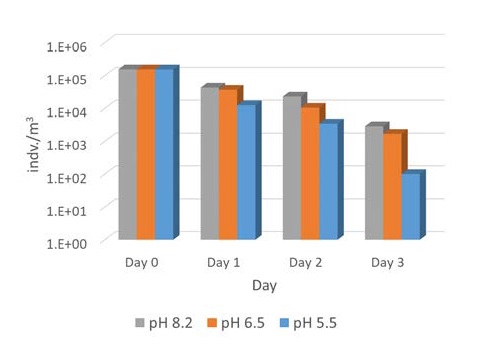
Inactivation of Artemia sp. with respect to different pH. The initial TRO concentration was 4 ppm and pH was controlled by CO2 injection
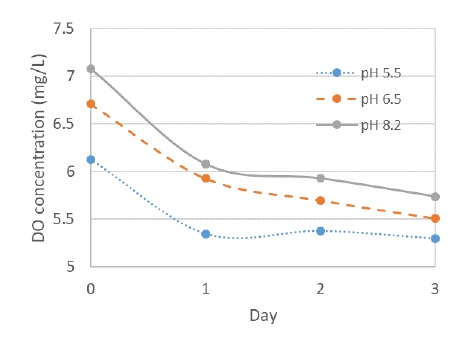
Figure 2 shows dissolved oxygen (DO) concentration as a function of pH. Overall, the DO concentration steadily decreased with increasing holding time and constantly decreased with decreasing pH, indicating that disinfection efficacy is related to DO concentration. There was no significant change in the concentration between 1 and 3 days of holding time at pH 5.5, implying that the enhanced inactivation of Artemia sp. on day 3 was not directly correlated with changes in DO concentration. Although TRO concentration decay was more noticeable in only electrochlorinated water than in electrochlorinated water with CO2 addition [4]. Our results did not show a drastic difference in the TRO concentration decay profiles (data not shown here). Further studies are needed to elucidate the cause of the enhanced disinfection efficacy.
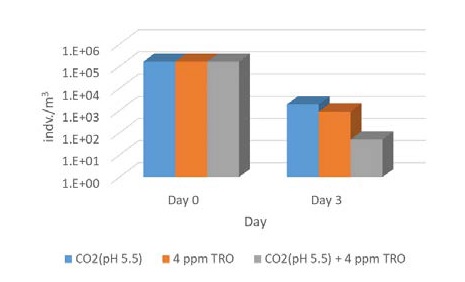
It is worthwhile to review the technique that was most effective for disinfection. As shown in Figure 3, the inactivation efficacy of Artemia sp. was 98.8%, 99.4%, and 99.9% in CO2 alone, 4 ppm TRO, and 4 ppm TRO + CO2 on day 3 of the holding time, respectively. From an engineering perspective, CO2 can be constantly injected to maintain a certain level of disinfection in ballast water tanks, and electrochlorination systems with CO2 addition can be used when entering the United States, where USCG ballast water treatment regulations are applied. Accordingly, it would be helpful to reduce the power consumption of BWTS.
Some BWTS manufacturers have attempted to use CO2 gas generation systems as the CO2 source. To our knowledge, there are no cases of direct application to BWTS using vessel exhaust gas. Therefore, it is important to verify if the exhaust gas emitted from a combustion engine can be applied to a BWTS. Table 1 shows the summary of auxiliary engine exhaust monitored. Maximum continuous rating (MCR) was 1,540 kW at a 75% engine load, and the exhaust gas flow rate was 7,066 kg/h. In addition, the CO2 concentration was approximately 5.62%.
When injected CO2 is dissolved in water, the concentrations of [H2CO3*], [HCO3-], and [CO32-] can be predicted using Equations (1)–(3). The total inorganic carbon (CT) is expressed by Equation (4), which is the sum of [H2CO3*], [HCO3-], and [CO32-].
| (1) |
| (2) |
| (3) |
| (4) |
The equilibrium constants for KHCO3- and KCO32- were analytically obtained using Equation (5) [7].
| (5) |
where T is the absolute temperature, and a1 to a5 are empirical constants.
constants. To calculate the pH inside the ballast water tank, the concentration of H+ was determined using Excel software. The dissolved inorganic carbon (DIC) concentration in the ballast water tank as a function of flow capacity is shown in Figure S2.
Table 2 shows the predicted pH inside the ballast water tank as a function of ballast water flow capacity. The ballast water flow capacity will be approximately 500 m3/h when the pH is 5.5, and it is indicated as the highest disinfection efficacy. A pH was predicted to be 5.9 when the ballast water flow capacity reached 1000 m3/h, that is, under conditions where vessels are frequently operated. From these results, a suitable pH for microorganism disinfection was achieved using only the vessel auxiliary engine without any acid additives.

Predicted pH in ballast water tank as a function of bal-last water flow capacity. Exhaust gas ratio was fixed to 50%, and the ballast water temperature was 22°C
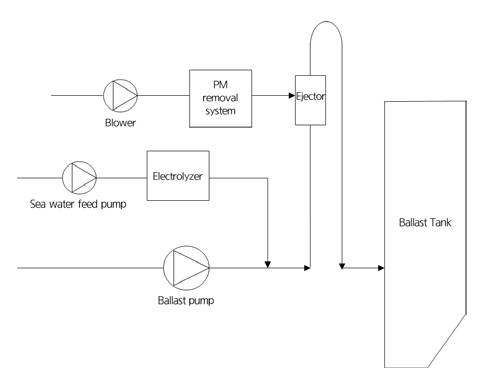
Finally, the IMO calls for the maritime industry to restrict its CO2 emissions by -40% by 2030 and -70% by 2050, compared to the base level in 2008 [8]. In view of the upcoming CO2 emission regulations, many shipbuilding manufacturers have begun researching the possibility of onboard carbon capture vessels. They have mainly focused on CO2 capture and storage using chemical absorbents. However, it will be difficult to apply to vessels owing to corrosion caused by chemical adsorbents and the expensive operation cost required to regenerate the chemical absorbent. However, if the injected CO2 in the ballast water tank is periodically neutralized during the voyage and discharged after attaining a pH similar to that of seawater, it is expected that CO2 can be partially reduced [9]. The proposed concept of an electrochlorination system combined with CO2 injection is illustrated in Figure 4.
4. Conclusions
We investigated the inactivation of Artemia sp. using an electrochlorination system combined with CO2 injection. At pH 5.5, the inactivation of Artemia sp. significantly increased by approximately 29 times compared to pH 8.4 on day 3 of holding time. Results showed that the main factors enhancing the disinfection efficacy were an increase in HOCl concentration with decreasing pH and a decrease in DO concentration due to the dissolution of CO2. Results of comparing disinfection efficacies revealed that the inactivation of Artemia sp. was 98.8%, 99.4%, and 99.9% in CO2 alone, 4 ppm TRO, and 4 ppm TRO + CO2 on day 3 of the holding time, respectively.
From an engineering perspective, it can be concluded that the CO2 emitted from a combustion engine can be directly used to reduce pH. Furthermore, if the injected CO2 in ballast water tanks is periodically neutralized during the voyage and discharged after attaining a pH similar to that of seawater, it is expected that CO2 can be partially eliminated. We hope that this study will provide insight into how to retrofit and design BWTS in the maritime industry.
Acknowledgments
This research was supported by the Korea Shipbuilding and Offshore Engineering Research Fund.
Author Contributions
Conceptualization, J. Joo; Investigation, D. H. Park; Data Curation, D. H. Park; Writing-Original Draft Preparation, J. Joo; Writing-Review & Editing, T-J. Rhee and J. H. Lee.
References
-
J. Joo, G. Jung, I. Oh, and T. Rhee, “Validation test on real scale UV reactor for ballast water treatment,” Environmental Engineering Research, vol. 26, no. 1, pp. 1-5, 2021.
[https://doi.org/10.4491/eer.2019.455]

-
W. A. Gerhard, K. Lundgreen, G. Drillet, R. Baumler, H. Holbech, and C. K. Gunsch, “Installation and use of ballast water treatment systems – Implications for compliance and enforcement,” Ocean and Coastal Management, vol. 181, no. 104907, pp. 1-9, 2019.
[https://doi.org/10.1016/j.ocecoaman.2019.104907]

-
B. Sayinli, Y. Dong, Y. Park, A. Bhatnagar, and M. Sillanpää, “Recent progress and challenge facing ballast water treatment – A review,” Chemosphere, vol. 291, part 2, pp. 1-23, 2022.
[https://doi.org/10.1016/j.chemosphere.2021.132776]

-
H. -G. Cha, M. -H. Seo, H. -Y. Lee, J. -H. Lee, D. -S. Lee, K. Shin, and K. -H. Choi, “Enhancing the efficacy of electrolytic chlorination for ballast water treatment by adding carbon dioxide,” Marine Pollution Bulletin, vol. 95, no. 1, pp. 315-323, 2015.
[https://doi.org/10.1016/j.marpolbul.2015.03.025]

-
C. M. Moffitt, B. Watten, A. Barenberg, J. henquinet, “Hydroxide stabilization as a new tool for ballast disinfection: efficacy of treatment on zooplankton,” Management of Biological Invasions, vol. 6, no. 3, pp. 263-275, 2015.
[https://doi.org/10.3391/mbi.2015.6.3.05]

-
T-L. T. Dang, T. Imai, T. V. Le, D-M. K. Nguyen, T. Higuchi, A. Kanno, K. Yamamoto, and M. Sekine, “Synergistic effect of pressurized carbon dioxide and sodium hypochlorite on the inactivation of Enterococcus sp. in seawater,” Water Research, vol. 106, pp. 204-213, 2016.
[https://doi.org/10.1016/j.watres.2016.10.003]

-
J. Šimůnek and A. J. Valocchi, “Geochemical transport,” Methods of Soil Analysis: Part 4 Physical Methods, pp. 1511-1536, 2002.
[https://doi.org/10.2136/sssabookser5.4.c62]

-
M. Buirma, J. Vleugel, J. Pruyn, V. Doedée, and D. Schott, “Ship-based carbon capture and storage: a supply chain fea-sibility study,” Energies, vol. 15, no. 3, pp. 1-21, 2022.
[https://doi.org/10.3390/en15030813]

- J. Joo, D. Park, T-J. Rhee, J. Lee, “Ballast water treatment system and ship having the same,” Korea, Patent 2021-0125158, September 17, 2021.