Possibility of military service-regeneration of LiOH for submarines and improvement in CO2 scrubbing performance of LiOH canisters
Copyright ⓒ The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Graphical Abstract
Abstract
Air management in a submarine environment is crucial. In particular, CO2 concentration management is directly associated with crew respiration. The CO2 scrubbing characteristics of LiOH are used to control the CO2 concentration inside a submarine; however, recently, the lithium requirement for manufacturing batteries used in electric vehicles has increased significantly, and the price of LiOH has increased accordingly. From the viewpoint of logistics support, the CO2 scrubbing efficiency of the LiOH canister for submarines must be improved, and the regeneration of Li2CO3 to LiOH should be considered. The X-ray diffraction and inductively coupled plasma atomic emission spectroscopy analyses of Li2CO3 discharged after use in the submarine confirm that impurities are extremely rare, and that impurities such as Si and Al are sufficiently removed via high-temperature heating. Therefore, in this study, Li2CO3 discharged after its actual use in a submarine is heated to a high temperature in an electric furnace to regenerate it to Li2O. This process is assessed based on differential thermal and thermo-gravimetric analyses, in addition to 99% Li2CO3; the findings show that the efficiency of the LiOH canister for submarines can be maximized by further reacting unreacted LiOH. Therefore, the existing static rectangular parallelepiped canister is changed to a rotating cylindrical canister, and changes in its performance in a closed environment with a CO2 atmosphere, such as a submarine, are examined. Using the modified canister, the CO2 scrubbing efficiency with only 15% less LiOH is higher than that of the existing canister.
Keywords:
Submarine, LiOH canister, LiOH regeneration, CO2 scrubbing efficiency, Type of canister1. Introduction
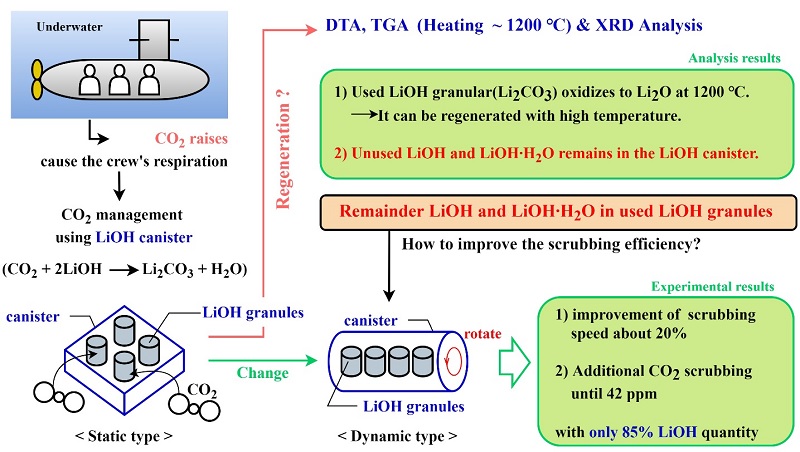
The air quality inside submarines that perform underwater operations, particularly the carbon dioxide (CO2) concentration, must be controlled. As the level of CO2 increases, the crew’s respiratory rate increases. High levels of CO2 can result in headaches, confusion, and eventual loss of consciousness. The CO2 emitted by the crew from underwater submarines may allow external air to flow into the room and ventilate when the submarine rises above the water. However, the ascent of submarines during missions and operations is limited significantly by the increased risk of operations due to location exposure. Therefore, in submarines, a method of chemically scrubbing CO2 using lithium hydroxide (LiOH) is used. In this process, LiOH is converted to lithium carbonate (Li2CO3), and the CO2 concentration in the submarine is maintained between 2,000 and 3,000 ppm on average. The use of LiOH in powder form may increase the CO2 scrubbing efficiency, but the leakage of LiOH particulates is detrimental to the human body; therefore, the granular type is used [1]-[2]. LiOH granules are packed into a rectangular parallelepiped canister that is statically loaded inside a ventilation cabinet to adsorb CO2. The LiOH canister cam be used to effectively adjust the CO2 concentration in spaces and conditions where ventilation is restricted, such as spacecraft [3], submarines, and deepsea divers, and several related studies have been conducted [4]-[8].
The price of LiOH used in submarines and that of Li2CO3 discharged by the use of submarines have increased by approximately 500% owing to the high demand for lithium batteries, as a result of the increase in electric vehicles. Additionally, the price of lithium is expected to increase in the future. Therefore, for the long term, Li2CO3 must be regenerated into LiOH, and the mechanism for reuse in submarines must be introduced. Research pertaining to LiOH regeneration has been conducted in the fields of chemistry and electricity, but not on ships and equipment [9]-[12]. In addition, the CO2 scrubbing performance of LiOH canisters for submarines currently in use must be analyzed; their use efficiency must be improved to reduce the use of LiOH.
In this study, when a submarine entered the home port after an underwater voyage, the LiOH canister that adsorbed CO2 formed due to the breathing of the crew was recovered; subsequently, analysis and improvement measures for this were examined. First, the regeneration potential of LiOH under high heat was examined using differential thermal analysis (DTA) and thermogravimetric analysis (TGA). After verifying the presence of unreacted LiOH inside the LiOH canister used based on the results of TDA and X-ray diffraction (XRD), we propose an improved canister for the further CO2 adsorption of unreacted LiOH. Next, we report the results of chamber experiments, such as the atmospheric condition of submarines, to confirm the change in CO2 scrubbing tendency as a result of using the improved canister.
The materials and figures related to security are not disclosed, and the used LiOH samples (Li2CO3) for the study are supported by the ROK navy submarine command.
2. CO2 Scrubbing in Submarines
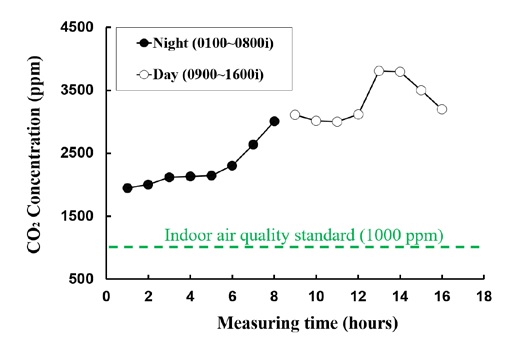
The CO2 concentration during the underwater voyage of a submarine is measured at 15 to 20 detection points depending on the submarine type; the measurement is controlled to avoid reaching the CO2 concentration limit of 1%. Figure 1 shows the CO2 concentration measurement results for 7 h during the day and 7 h at night during the actual underwater voyage of the submarine.
During the underwater voyage of the submarine, the average CO2 concentration is approximately 3400 ppm during the day and 2450 ppm at night. The high CO2 concentration during the daytime is due to the activity of the crew. Owing to the characteristics of the submarine, which was operated in three shifts, two-thirds of the crew were sleeping (15ℓ / h∙person), and the remaining one-third was on duty (18-24ℓ / h∙person). Therefore, the CO2 concentration during dawn hours increased gradually from low levels. However, it remained at a high level during the day when the entire crew was engaged in activities such as training and maintenance (24–90/ h∙person). Additionally, the increase in the CO2 concentration was significant.
The decrease in CO2 concentration between 9 and 11 h and between 13 and 16 h was due to CO2 scrubbing by the LiOH canister (Figure 2).
CO2 from the crew’s respiration can be chemically scrubbed from a submarine’s atmosphere via the following overall reaction with LiOH:
When this reaction proceeded to completion, 0.919 g of CO2 reacted with each gram of LiOH. These data are termed the stoichiometric limits of the reaction. Furthermore, this reaction is highly exothermic, i.e., the temperature of LiOH crystals increases as they react with water vapor and CO2, releasing 21.4 kcal per mole of CO2 scrubbed [9]. However, these temperature changes are not important for air management in submarines.
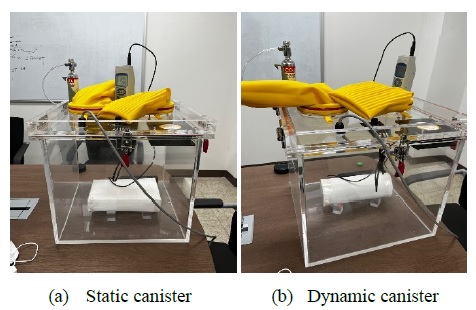
The LiOH canister (Figure 2), an important element for CO2 adsorption, used in this study is based on the same principle as the astronaut respiratory CO2 removal (Figure 3) in the Apollo 13 project. In fact, the same mechanism was used previously. LiOH powder is a strong alkaline substance and is toxic to the human body when inhaled; therefore, LiOH powder must not flow out of the canister. During the Apollo 13 project, LiOH granules were wrapped in the endothelium and stored in a canister (Figure 3); this is similarly performed in canisters currently used in submarines [3]. Meanwhile, in nuclear submarines that operate regenerative CO2 scrubbers, the passive method using LiOH is operated as a backup plan. The navy of some countries, such as the UK, operates LiOH in the form of a suspending curtain [9].
3. DTA, TGA, and XRD results for LiOH
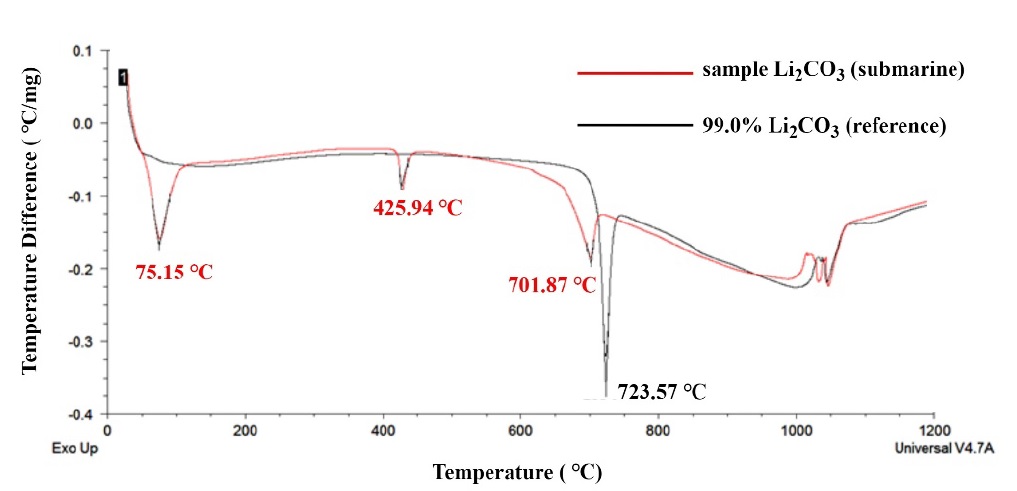
The results of DTA, TGA, and XRD of LiOH granules (Li2CO3) discharged after their use on a submarine are reported in the section. The sample used in this study was LiOH granules discharged after use on a ROK navy submarine that were further exposed to CO2 for approximately 60 d under submarine atmospheric conditions for better CO2 adsorption. Using DTA and TGA, Li2CO3 with a purity of 99.0% was analyzed as a comparative substance. Figure 4 shows the DTA results of the granular LiOH sample after its use in a submarine.
As a result of heating the sample to 1200 °C in an electric furnace, two sections of endothermic reaction were observed in the sample (red line), in which the reference substance (99.0% Li2CO3) was not observed prior to the melting point (723 °C). First, the endothermic reaction observed at approximately 75.15 °C was manifested by the H2O vaporization of the water in the sample, i.e., LiOH∙ H2O. The endothermic reaction in the second section occurred at approximately 425.94 °C owing to the arrival of some unreacted LiOH in the sample at the melting point. The theoretical melting point of LiOH is 445 °C. The second endothermic reaction indicates that some LiOH remained in the sample. In other words, the LiOH canister used in the submarine could not utilize all the internal LiOH granules, and the remaining were discharged. Beginning from 700 °C, the sample showed almost the same reaction mode as the reference; additionally, CO2 was separated from Li2CO3 and discharged, whereas Li2O was generated.
Li2O is formed in a vitrified state; when it reacts with distilled water, LiOH∙H2O is formed. When LiOH∙H2O is heated to approximately 150 °C and dried, LiOH is formed [13]. Prior to the formation of LiOH, it is advantageous to produce its granular form using LiOH∙H2O.
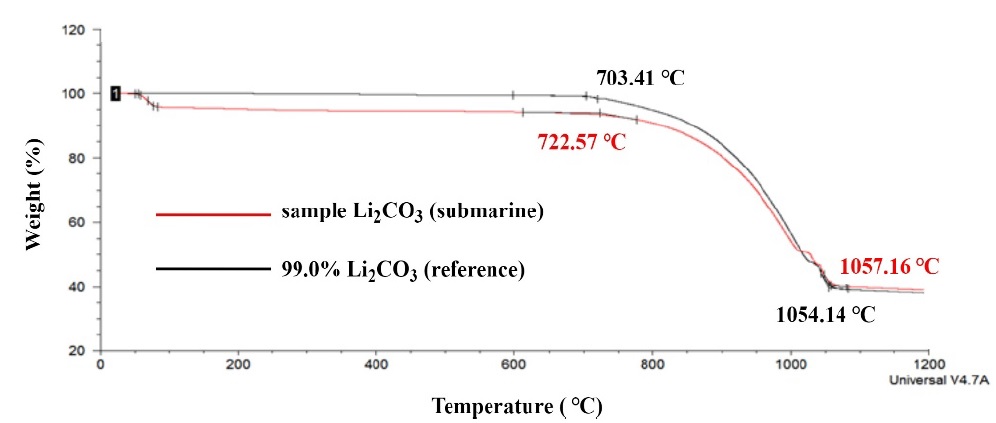
Figure 5 shows the TGA results for the LiOH granules used. The results show a decrease in weight due to the vaporization of H2O at approximately 75 °C. However, no change was observed at approximately 425 °C, where a secondary decrease was observed in the DTA. This is because the mass difference between hydrogen and lithium is slight during the melting of unreacted LiOH into Li2O. Subsequently, in the Li2O formation process at 700 °C or higher, the reference and sample show the same tendency in weight change. The TGA results quantitatively complement the DTA results.
The results of DTA and GTA confirmed that the LiOH used, i.e., Li2CO3, which absorbed CO2 generated in the crew’s respiratory process, can be heated to 1200 °C and regenerated to Li2O.
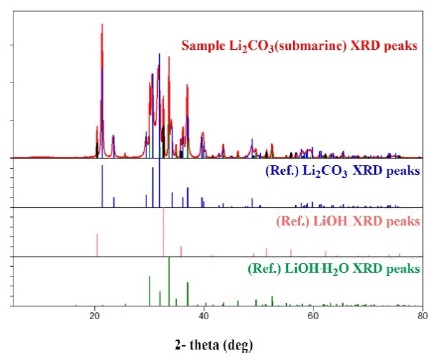
Subsequently, XRD pattern analysis was performed on the LiOH samples. The XRD pattern shows a plot of the intensity of X-rays scattered at different angles by the sample. Copper K-α was used as the X-ray energy for the analysis. The XRD pattern analysis was commissioned by the Korea Advanced Institute of Technology. The LiOH canister installed in submarines is used only to adsorb CO2 emitted by the crew’s breathing; therefore, the substances formed after its use are primarily Li, O, H, and C compounds, whereas impurities are slight. Only the diffraction peaks of LiOH, LiOH∙H2O, and Li2CO3 were confirmed in the XRD results (Figure 6) of the discharged granular LiOH sample. constituted 97% of the metallic substance, whereas other substances constituted the remaining 3% and can be removed via high-temperature heating.
The following conclusions were obtained based on a comprehensive analysis of the DTA, TGA, and XRD results: First, the Li2CO3 discharged after use in the submarine indicated a high-purity lithium content that can be regenerated when heated at high temperatures. Second, when heated to a high temperature of 1200 °C or higher, the discharged Li2CO3 granules were regenerated to Li2O, which is the base material of LiOH. Unreacted LiOH was present in the LiOH canister used in the process. Third, even when the CO2 reaction conditions were sufficient, the scrubbing efficiency of the LiOH granules could not be guaranteed when using the existing canisters. Therefore, canisters with new forms and methods that can maximize the use of unreacted LiOH, as identified via TGA and XRD patterns, must be developed.
4. Improvement in CO2 Scrubbing Efficiency
Manufacturing costs for LiOH canisters used in submarines increased by more than 330% as of 2018. This is because of the significant increase in the price of battery raw materials (LiOH and Li2CO3) owing to the increasing demand for electric vehicles worldwide. This trend is expected to continue in the near future.
Therefore, improving the efficiency of the canister via the further adsorption of CO2 by unreacted LiOH in the canister can be an considered to reduce the submarine operating costs incurred for purchasing LiOH. Thus, we propose changing the canister type from static to dynamic
To verify the effect of the canister in the dynamic state, we constructed an experimental equipment, as shown in Figure 7. A static canister, whose size is one-half that of the canister used in an actual submarine, was prepared by filling a canister completely with unused LiOH. Next, a closed chamber with submarine atmospheric conditions was constructed, and a scrubbing test was performed on a static LiOH canister under a CO2 concentration of 5000 ppm in the chamber (Figure 7(a)). Subsequently, as shown in Figure 7(b), after preparing a cylindrical dynamic canister with the same volume as the static canister, it was filled to 85% in volume with LiOH granules. The remaining 15% free space allows the particulates to mix internally as the canister is rotated, and the dynamic canister was rotated 90° once every 30 s. The inside of each canister corresponds to a completely empty space with no media or grid, and the shell of the canister is constructed using a double H14 grade high-efficiency particulate air filter (> 0.3 μm). The chamber was completely sealed. CO2 measurements were performed using a GC-2028 CO2 measuring instrument, and a fan was mounted inside the chamber to circulate the internal air.
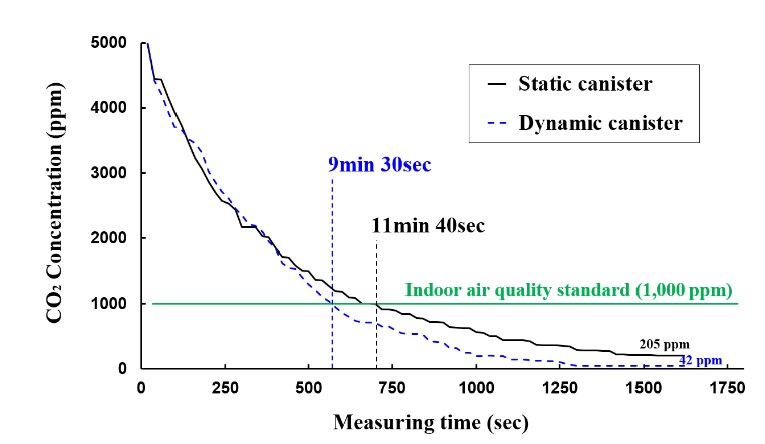
Figure 8 shows the results of the CO2 scrubbing tests on static and dynamic canisters. Both canisters began CO2 scrubbing at 5000 ppm, which is the initial condition of the closed air chamber. The experiment was terminated when the CO2 concentration in the chamber stabilized.
The experimental results show that the static canister required 11 min and 40 s to attain the indoor air quality standard of 1000 ppm, whereas the dynamic canister required 9 min and 30 s. The static canister stopped scrubbing when the residual CO2 concentration in the chamber was 205 ppm, whereas the dynamic canister performed additional scrubbing until the residual CO2 concentration reached 42 ppm. Because the LiOH granules inside the dynamic canister were only 85% of those in the reference static canister, the performance improved (20% and 4% improvements in the scrubbing speed and scrubbing rate, respectively) while 15% of LiOH was conserved when using the rotating dynamic canister.
Considering the high market price of lithium products owing to the rapid increase in demand for electric vehicle batteries, the 15% reduction in LiOH usage affords an annual savings of $1 million from the viewpoint of naval logistics management. In addition, it results in a higher LiOH stock rate and reduces the submarine operation time.
4. Conclusion
In this study, LiOH granules used to maintain the concentration of CO2 generated by the crew’s breathing in a submarine were analyzed via DTA, TGA, and XRD patterns. The LiOH granules (Li2CO3) discharged from the submarines contained minute impurities. Therefore, regeneration via heating was sufficiently effective.
The DTA and TGA results for LiOH granules (Li2CO3) discharged after use confirmed that when Li2CO3 was heated to 1200 °C, CO2 was removed. Additionally, Li2O generated during heating reacted with distilled water to yield LiOH∙H2O. The DTA results confirmed, the absence of LiOH melting in the reference Li2CO3, thus confirming that unreacted LiOH that did not adsorb CO2 was present inside the fully used LiOH granules and canister.
Therefore, to maximize the CO2 scrubbing efficiency of LiOH granules, a cylindrical rotating dynamic canister was proposed. After considering its effect experimentally, the dynamic canister was further improved based on the 85% LiOH content of the static canister.
Currently, based on the verified results, studies are performed to mass-regenerate Li2CO3 discharged from submarines. These studies will be continued such that the ROK navy submarine unit can demonstrate its own military-serviced regeneration capability in the future.
Abbreviations
| DTA : | Differential thermal analysis |
| TGA : | Thermogravimetric analysis |
| XRD : | X-ray diffraction |
| CO2 : | Carbon dioxide |
| LiOH : | Lithium hydroxide |
| LIOH∙H2O : | Lithium hydroxide monohydrate |
| Li2CO3 : | Lithium carbonate |
Acknowledgments
This study was supported by the 2022 Academic Research Project of the Naval Institute for Ocean Research of the Republic of Korea Naval Academy.
Author Contributions
Conceptualization, H. M. Baek; Methodology, H. M. Baek; Investigation, H. M. Baek; Writing-Original Draft Preparation, H. M. Baek; Writing-Review & Editing, H. M. Baek; Visualization, H. M. Baek; Supervision, H. M. Baek; Project Administration, H. M. Baek.
References
- K. Ikeda, and et al., “Comparison of compound a production by three different carbon dioxide absorbents,” 2010 ASA Abstract 451475.
- T. E. Dahms, N. Statzer, and G. R. Haynes, “Evaluation of a new carbon dioxide absorber: Litholyme,” Abstract A 717, ASA Meeting, Oct 17, 2010.
- Most famous CO2 absorber in the world: The Story of Apollo 13, www.howequipmentworks.com, , Accessed April 21, 2022.
- J. Anthony, and et al., “Implemetation of lithium hydroxide as a dual CO2/H2O scrubber for a rodent research life support system,” 48th International Conference on Environmental Systems, 8-12 July 2018, Albuquerque, New Mexico.
- J. R. Jaunsen, The behavior and capabilities of lithium hydroxide carbon dioxide scrubbers in a deep sea environment,” Annapolis, United States Naval Academy, U.S. Tri-dent Scholar Committee, 1989.
- M. P. Dorsch, The Anesthesia Gas Machine, www.ud-mercy.edu/crna/agm/07.htm, , Revised July 2012.
-
P. Zilberman, “The CO2 absorber based on LiOH,” Acta Mearisiensis – Seria Medica, vol. 61, no. 1, pp. 4-6, 2015.
[https://doi.org/10.1515/amma-2015-0023]

- P. L. Perrotta, A. Davis, and A. McCarrick, “Storage stability of lithium hydroxide used in the submarine force,” U.S. Naval submarine medical research laboratory Report 1190, 23 October, 1993.
- J. -E. Park, M. -H. Park, H. -J. Seo, T. -S. Kim, D. -W. Kim, B. -R. Kim, and H.-L. Choi, A study on the pyrolysis of lithium carbonate for conversion of lithium hydroxide from lithium carbonate, Journal of the Korean Crystal Growth and Crystal Technology, vol. 31, no. 2, pp. 89-95, 2021 (in Korean).
-
Q. Zhao, L. Hu, W. Li, C. -J. Liu, M. Jiang, and J. -J. Shi, “Recovery and regeneration of spent lithium-ion batteries from new energy vehicles,” Frontiers in Chemistry, vol. 8, 2020.
[https://doi.org/10.3389/fchem.2020.00807]

- D. -W. Kim, J. -R. Park, N.- K. Ahn, G. -M. Choi, Y. -H. Jin, and J. -K. Yang, “A review on the recovery of the lithium carbonate powders from lithium-containing sub-stances,” Journal of the Korean Crystal Growth and Crystal Technology, vol. 29, no. 3, pp. 91-106, 2019 (in Korean).
-
P. Zhang, T. Yokoyama, O. Itabashi, T. M. Suzuki, and K. Inoue, “Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries,” Hydrometallurgy, vol. 47, no. 2-3, pp. 259-271, 1998.
[https://doi.org/10.1016/S0304-386X(97)00050-9]

- W. Norfleet and W. Horn, “Carbon dioxide scrubbing capabilities of two new non-powered technologies,” U.S. Naval submarine medical research laboratory Report No. TR 1228, 17 August, 2003.