Effects of Na2B4O7·10H2O on microstructure and mechanical properties of AlSi7Mg0.3and AlSi10MnMgAlSi10MnMg alloys
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Iron contained in aluminum alloys is an impurity that cannot be removed, and it causes porosity in the solidification process and forms intermetallic compounds, which negatively affects mechanical characteristics; therefore, Fe intermetallic compound control is required. Therefore, in this study, an analysis of the microstructure and mechanical properties of aluminum alloys produced by injecting Na2B4O7·10H2O with a maximum solubility of hydrogen was conducted. After the addition of Na2B4O7·10H2O, the size of the Fe-rich intermetallic AlSi7Mg0.3 compound reduced, and the needle-shaped Fe intermetallic phase transformed into Chinese-script-shaped in the AlSi10MnMg alloy. Further, the fraction of the Fe intermetallic compounds reduced by 0.17 % and 0.15 % in AlSi7Mg0.3 and AlSi10MnMg, respectively, after the addition of Na2B4O7·10H2O, and the secondary dendrite arm spacing and size of the Si particles increased. In the alloys with the addition of Na2B4O7·10H2O, the increased secondary dendrite arm spacing and blocky Si reduced the tensile strength of the AlSi7Mg0.3 and AlSi10MnMg alloys to 11.45 MPa and 11.78 MPa, respectively. In contrast, the transformed Fe intermetallic compounds increased the hardness of the Al matrix to 4 HV and 2.53 HV in AlSi7Mg0.3 and AlSi10MnMg alloys, respectively.
Keywords:
Na2B4O7 ·10H2O, Fe intermetallic compound, AlSi7Mg0.3, AlSi10MnMg, Microstructure1. Introduction
Commercially pure aluminum is an extremely flexible metal with low strength, and is not used for structural applications [1] however, it is utilized by alloying with Si, Mg, Cu, Zn, etc. [2]-[5]. Casting defects, such as intermetallic compounds and porosity occur during casting when an alloying element is added to a master alloy [6]-[8]. Casting defects must be eliminated because they are a major cause of unexpected damage and fracture, negatively affecting the quality of the cast product [9]-[11].
Fe is an impurity that cannot be removed from pure aluminum has a very low solid solubility of up to 0.05 % in aluminum [12] and is bonded with Si, Mn, or it is precipitated as an intermetallic compound with brittleness [13]-[14]. Brittle plate-like Fe intermetallic compounds act as nucleation sites for porosity [15], preferential corrosion attack, or as stress concentration and weak points [16]-[18], negatively affecting the mechanical properties of castings. The needle-shaped Fe-rich intermetallic and porosity have a problem with AlSi7Mg0.3 and AlSi10MnMg alloys containing 0.2 % or less of Fe in an Al-Si-based alloy that occupies most of the casting alloys [19]-[21]. To circumvent these casting defects, degassing and flux processes [22] or heat treatments are performed to transform the shape of the Fe intermetallic compounds or the cast alloy is re-dissolved in the Al matrix [23].
When Na2B4O7·10H2O is used as hydrogen [13]-[14], [24] or aluminum brazing fluxes [25], and as an electrolytic solution for the plasma electrolyte oxidation (PEO) processes [26], Na and B components are added for eutectic Si and grain refinement [27]-[28], respectively, but they cause porosity due to the O component. However, previous studies have shown that Na2B4O7·10H2O reduces the iron contents from 0.14 wt.% to 0.1 wt.% and 0.33 wt.% to 0.18 wt.%, i.e. by more than 45 % in pure aluminum [13]-[14]. Despite the positive effects of Na2B4O7·10H2O, studies on the effects of aluminum alloys on iron intermetallic compounds and microstructures are insufficient. In addition, aluminum alloys produce numerous intermetallic compounds than pure aluminum [20], therefore, to analyze the effects of Na2B4O7·10H2O on aluminum alloys further studies are required. In this study, Na2B4O7·10H2O was used to confirm the intermetallic compounds formed by the maximum hydrogen solubility in aluminum alloys, to analyze the mechanical properties, and to control the microstructures to collect data for Na2B4O7·10H2O applications.
2. Experimental Methods
2.1. Materials
AlSi7Mg0.3 and AlSi10MnMg alloys were used to investigate the effects of the hydrogen flux on aluminum alloys.
The chemical composition of AlSi7Mg0.3 alloy is 7.06 wt.% Si, 0.36 wt.% Mg, 0.02 wt.% Mn, 0.13 wt.% Fe, 0.06 wt.% Cu, 0.09 wt.% Ti, and 92.28 wt.% Al. Few other chemical elements constituted less than 0.01 wt.% (Ni, Cr, Pb). The chemical composition of AlSi10MnMg alloy is 10.11 wt.% Si, 0.32 wt.% Mg, 0.6 wt.% Mn, 0.06 wt.% Fe, 0.08 wt.% Ti, 0.02 wt.% Sr, and 88.81 wt.% Al. Few other chemical elements in AlSi10MnMg alloy constituted less than 0.01 wt.% (Cu, Cr, Ni, Pb). Na2B4O7·10H2O hydrogen flux with a maximum hydrogen solubility of 24.08 ppm was added to the aluminum alloy. The alloy was dissolved at a temperature of 800 °C, and Na2B4O7·10H2O was added after 30 min of degassing, followed by stirring for 5 min, and then injected into the mold.
2.2 Thermodynamic simulations
The thermodynamic calculations of intermetallic compounds produced in the range of 25–800 °C were performed using JmatPro®oftware, on as-cast AlSi7Mg0.3, as-cast AlSi10MnMg, AlSi7Mg0.3 alloys with Na2B4O7·10H2O, and AlSi10MnMg alloys with Na2B4O7·10H2O. The intermetallic compounds were produced with AlSi7Mg0.3 and AlSi10MnMg alloys.
2.3. Microstructure analysis
The specimens were polished using SiC polishing papers of up to #2400 grit to observe the microstructure of the alloy. In addition, the specimens were polished using a 0.25 μm diamond and a 0.04 μm colloidal suspensions. The porosity area fraction of the reduced pressure test specimens, microstructure and secondary dendrite arm spacing of the pretreated specimen, and fracture surfaces were examined by field emission scanning electron microscopy (FE-SEM; MIRA3, Tescan) with an accelerating voltage of 15.0 kV and using an optical microscope. To analyze the crystal structure of the as-cast and Na2B4O7·10H2O-added alloys, X-ray diffraction (XRD, D/MAX 2500 VL/PC, Rigaku) analysis was performed in the range of 20°–90° using Cu Kα radiation, operating at 40 kV and 30 mA.
2.4. Mechanical property analysis
The mechanical properties of the alloys were evaluated to measure the tensile strength, yield strength, elongation, and hardness. A tensile test piece was prepared according to the ASTM E8 sub-size sheet tensile test.
The tensile test of each specimen was conducted for four trials using a UNIVERSAL TESTING MACHINE KDMT-156 at a cross-head rate of 1 mm/min. For the micro hardness test, an HM-122 hardness test machine (Akashi Co., Tokyo, Japan) was used, and the load was set to 1 N with an indentation time of 10 s, and the hardness of the specimens was recorded for 10 trials each.
3. Results and Discussion
3.1 Thermodynamic simulations
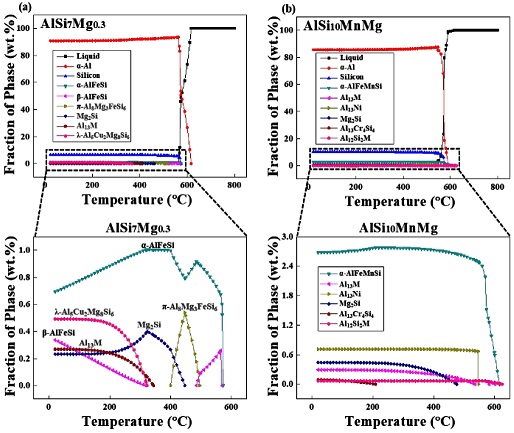
Figure 1 shows the thermodynamic simulation of the ASTM AlSi7Mg0.3 and AlSi10MnMg alloys calculated using the JmatPro software program. When analyzing the solidification behavior of each alloy based on Figure 1, the formation of the α-Al phase begins at approximately 600 °C in the AlSi7Mg0.3 alloy. As the solidification proceeded, a monovalent reaction of L → α-Al + Si proceeded at approximately 570 °C, and β-AlFeSi was formed from the reaction L→ α-Al + Si + β-AlFeSi. Therefore, the phase fraction decreases in the order of α-Al8Fe2Si to Al5Cu2Mg8Si6, β-Al5FeSi, Mg2Si, and Al3M. The AlSi10MnMg alloy formed an α-Al phase at 600 °C, and a monovalent reaction progressed at 570 °C. A considerable amount of α-AlFeMnSi is formed; however, the fractions of Al13Ni, Mg2Si, Al3M, Al13Cr4Si4, and Al12Si2M phases are reduced. In Al3M and Al12Si2M, M denotes a transition metal in the thermodynamic simulations of AlSi7Mg0.3 and AlSi10MnMg alloys. Transition elements such as Fe, Cu, Mn, Ni, and Zn are less soluble, and most of them precipitate.
3.2 Microstructure of aluminum alloys
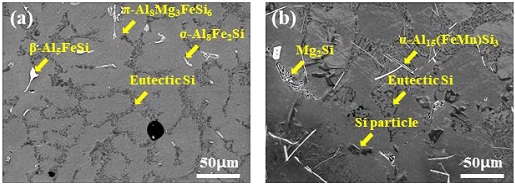
The microstructure and intermetallic compounds of the alloy before and after the addition of Na2B4O7·10H2O were examined using FE-SEM and energy-dispersive spectroscopy (EDS) to compare the theoretically calculated intermetallic compounds through JmatPro with the produced phases, as shown in Figure 2.
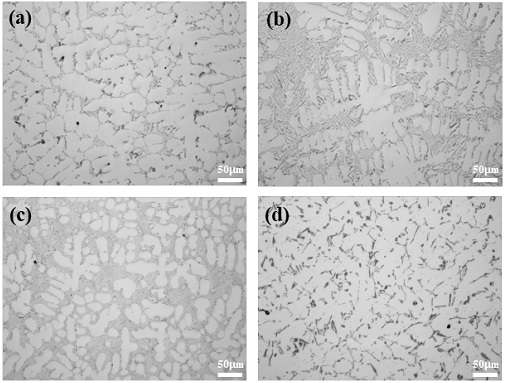
In Figure 2, the presence of the α-Al matrix, eutectic Si, α-Al15(FeMn)Si3, β-Al5FeSi, and α-Al8Fe2Si phases were confirmed, and π-Al8Mg3FeSi6 that should disappear at 400 °C was observed for AlSi7Mg0.3. In AlSi7Mg0.3 and AlSi10MnMg alloys, dendrites, a typical casting microstructure, were observed, and in addition, α-Al dendrites and fine Al-Si eutectic phases were identified. In particular, needle-shaped β-Al5FeSi and α-Al15(FeMn)Si3 were observed in AlSi7Mg0.3 and AlSi10MnMg, respectively. These needle-shaped intermetallic compounds have a negative effect on the mechanical properties by causing brittleness due to their weak bonding force with the matrix [15]-[18]. Further, Cu, Ni, and Cr intermetallic compounds which are present in Figure 1 are not observed in Figure 2 because they are present only in negligible amounts in AlSi7Mg0.3 and AlSi10MnMg alloys.
However, as shown in Figure 3, the size of β-Al5SiFe decreased after the addition of Na2B4O7·10H2O. In the case of AlSi10MnMg, the needle shaped α-Al15(FeMn)Si3 was converted to Chinese-script Chines-script compound, which indicates that the structure modified through the heat-treatment process [23].

SEM images (magnified 3,000×) of (a) as-cast AlSi7Mg0.3, (c) as-cast AlSi10MnMg, (b) AlSi7Mg0.3 with Na2B4O7, and (d) AlSi10MnMg with Na2B4O7
Figure 4 shows the microstructure of the aluminum alloys observed using an optical microscope and shows the secondary dendrite arm before and after the addition of Na2B4O7·10H2O. AlSi7Mg0.3 with Na2B4O7, and AlSi10MnMg with Na2B4O7 have blocky coarsened Si compared to the as-cast alloy.
SEM images (magnified 200×) of (a) as-cast AlSi7Mg0.3, (c) as-cast AlSi10MnMg, (b) AlSi7Mg03 with Na2B4O7, and (d) AlSi10MnMg with Na2B4O7
From Figure 5(a), the AlSi7Mg0.3 alloy has secondary dendrite arm spacing of 25.95 (± 2.88) μm; however, it increased to 29.77 (± 3.53) μm when Na2B4O7 is added, and in AlSi10MnMg with Na2B4O7, the SDAS value increased from 24.86 (± 3.88) μm to 27.64 (± 4.49) μm. In addition, from Figure 5(b), adding Na2B4O7 to AlSi7Mg0.3 reduced the fraction of Fe intermetallic compounds from 1.11 % (± 0.33 %) to 0.94 % (± 0.24 %), and in the case of AlSi10MnMg, the fraction decreased from 1.02 % (± 0.31%) to 0.87 % (± 0.23%). Further, B in Na2B4O7 was bound to Fe and precipitated into another phase of Fe2B [13]-[14] therefore, the fractions of Fe intermetallic compounds of β-AlFeSi, α-AlFeSi, and α-Al(FeMn)Si were reduced. The amount of needle-shaped Fe intermetallic phases, which are positioned at the tip of the α-Al phase and intermetallic compounds, is reduced, consequently, SDAS values increased [29].
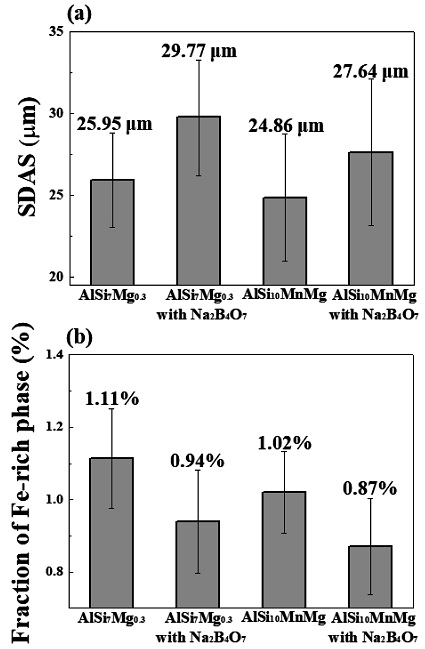
(a) Secondary dendrite arm spacing and (b) fraction of Fe-rich intermetallic phases of as-cast AlSi7Mg0.3, as-cast AlSi10MnMg, and alloys with the addition of Na2B4O7
As shown in Table 1, the area fraction of Si increased from 11.97 % (± 2.12%) to 12.15 % (± 1.12%) and from 12.18 (±1.18%) to 12.53 (± 2.01%) for AlSi7Mg0.3 and AlSi10MnMg, respectively. Further, the Si sphericity of AlSi7Mg0.3 and AlSi10MnMg decreased from 0.805 (± 0.04) and 0.824 (±0.02) to 0.795 (±0.04) and 0.752 (±0.03), respectively, after adding Na2B4O7. This changed shape is inferred from the Ostwald ripening effects, wherein, the number of eutectic grains generating eutectic Si nucleation is increased due to Na2B4O7. Therefore, the surface area between the liquid and solid is increased, consequently reducing the nucleation rate [30].
3.3 XRD analysis
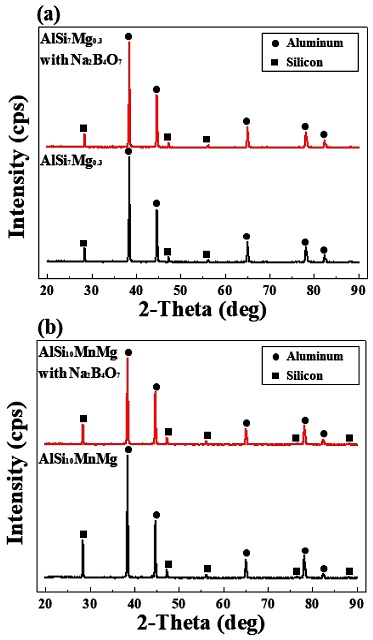
Figure 6 shows the XRD patterns of AlSi7Mg0.3 and AlSi10MnMg before and after the addition of Na2B4O7. The analysis confirmed that the main peaks of the detected crystalline phases were peaks of Al and Si in the Al-Si alloys. Because the intermetallic compounds observed using FE-SEM and EDS have a finer amount of precipitation than aluminum and silicon, they are present as background noise and were not detected as XRD peaks [31], and the XRD patterns of both AlSi7Mg0.3 with Na2B4O7 and AlSi10MnMg with Na2B4O7 alloys have no significant difference.
3.4 Porosity analysis
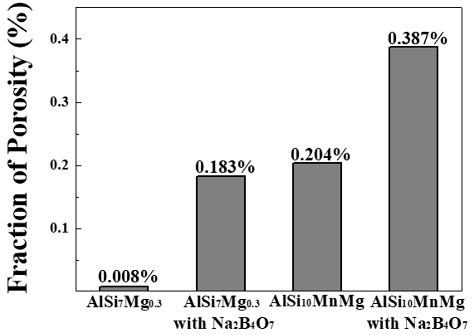
Figure 7 shows the porosity area fraction of as-cast AlSi7Mg0.3, as-cast AlSi10MnMg and alloys with the addition of Na2B4O7 by conducting a reduced pressure test. AlSi7Mg0.3 showed a porosity fraction of 0.008 %, and when Na2B4O7 was added, the fraction increased by 0.17 % to a value of 0.183 % and that of AlSi10MnMg increased by 0.18 % from 0.204 % to 0.387 %. Hydrogen in both Na2B4O7 and molten metal is dissolved in aluminum, and then supersaturated hydrogen molecules are formed between dendrites by exceeding the hydrogen solubility during solid-phase solidification in the liquid phase.
3.5 Tensile test and micro-hardness of aluminum alloy
Table 2 shows the changes in the tensile characteristics of alloys with the addition of Na2B4O7. When Na2B4O7 was added, the tensile strength of AlSi7Mg0.3 alloy decreased from 176.32 (± 16.8) MPa to 164.87 (± 7.15) MPa, and that of AlSi10MnMg alloy decreased from 195.11 (± 8.42) MPa to 183.33 (± 9.2) MPa. The yield strength of AlSi7Mg0.3 and AlSi10MnMg alloys reduced from 128.85 (± 6.77) MPa to 119.65 (± 5.49) MPa and from 131.16 (± 4.91) MPa to 126.67 (± 4.33) MPa, respectively. The tensile strength and yield strength were reduced after the addition of Na2B4O7 in both the alloys and the pore area fraction and widened SDAS increased after the addition of Na2B4O7 as afore-mentioned. In the case of the elongation, AlSi7Mg0.3 had an elongation of 9.89 (± 1.56) %, and the elongation increased to 11.28 (± 0.85) % after the addition of Na2B4O7. However, the elongation of AlSi10MnMg decreased from 15.76 (± 2.82) % to 14.06 (± 1.74) % after the addition of Na2B4O7.

Yield strength, tensile strength, and tensile strain of ascast AlSi7Mg0.3, as-cast AlSi10MnMg, and alloys with the addition of Na2B4O7
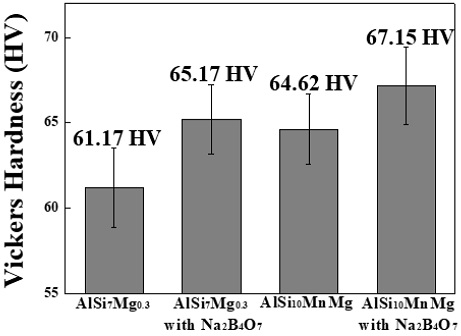
Further, Figure 8 shows that when Na2B4O7 is added, the hardness characteristics of AlSi7Mg0.3 increased from 61.17 (±2.34) HV to 65.17 (±2.04) HV and that of AlSi10MnMg also increased from 64.62 (±2.07) HV to 67.15 (±2.25) HV, which are in contrast to the tensile strength results. Although the addition of Na2B4O7·10H2O causes high porosity, brittle and needle-shaped intermetallic compounds are converted into small intermetallic compounds or Chinese-script-shaped intermetallic compounds to increase the bonding strength with an Al matrix, thereby increasing the hardness value. This is caused by the decrease in the area fraction of Fe intermetallic compounds that negatively affect the hardness characteristics [32]-[33].
3.6 Fracture surface of aluminum alloy
Figure 9 shows the fracture surfaces of AlSi7Mg0.3 and AlSi10MnMg alloys after the tensile test, and all fracture surfaces contain dimple shapes and ductility failures, which are broken by micro-voids and primary Si particles, which is the main fracture mechanism [34]. As shown in Figure 9, fracture occur in the dendrite direction in AlSi7Mg0.3, and AlSi7Mg0.3 with Na2B4O7 has dendrites inside the pores, which are microvoids in the form of shrinkage pores occurring during the phase shift of the solid. In other fracture locations, AlSi7Mg0.3 consisted of fracture of the eutectic Si particles and ductile fracture. In AlSi10MnMg, fracture occurred due to relatively larger and more voids than other alloys [34], and the AlSi10MnMg with Na2B4O7 alloy fractured due to blocky Si, and cleavage fracture was observed near the pores.
4. Conclusion
Fe-rich intermetallics of π-Al8Mg3FeSi6 and β-Al5SiFe were observed in AlSi7Mg0.3, but small and fine intermetallic compounds were observed after Na2B4O7 addition. Although α-Al15(Fe,Mn)Si2 and eutectic Si particles were observed in AlSi10MnMg, the needle-shaped α-Al15(Fe,Mn)Si2 transformed to Chinese-script-shaped α-Al15(Fe,Mn)Si2 with the addition of Na2B4O7. Further, the SDAS of AlSi7Mg0.3 increased to 3.82 μm after the addition of Na2B4O7, and that of AlSi10MnMg increased to 2.69 μm. The Fe intermetallic phase fraction of AlSi7Mg0.3, as-cast AlSi10MnMg alloys decreased by 0.17 % and 0.15 %, respectively, despite the addition of Na2B4O7 with maximum hydrogen solubility. The tensile strength and yield strength of the AlSi7Mg0.3 with Na2B4O7 alloy decreased to 11.45 MPa and 9.2 MPa, and those of AlSi10MnMg with Na2B4O7 decreased to 11.78 MPa and 4.5 MPa, respectively. However, when Na2B4O7 was added, the elongation of AlSi7Mg0.3 and AlSi10MnMg alloys increased by 1.34 % and decreased by 1.7 %, respectively. Despite the decrease in the tensile values, the hardness values of AlSi7Mg0.3 and AlSi10MnMg increased by 4 HV and 2.53 HV when Na2B4O7 was added. Therefore, the Na2B4O7 in the alloys functions as a flux and in addition generates a microstructure control effect, suggesting the prospect of Na2B4O7-based applications.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) granted by the Korean Government (NRF-2019R1I1A3A01062863).
Author Contributions
Conceptualization, J. Bang and E. Lee; Methodology, J. Bang; Software, J. Bang; Validation, J. Bang, E. Byon and E. Lee; Formal Analysis, J. Bang; Investigation, J. Bang; Resources, J. Bang; Data Curation, J. Bang; Writing—Original Draft, Preparation, J. Bang; Writing—Review & Editing, E. Byon and E. Lee; Visualization, J. Bang; Supervision, E. Lee; Project Administration, E. Lee; Funding Acquisition, E. Lee. All authors have read and agreed to the published version of the manuscript.
References
- T. H. G. Megson, Introduction to Aircraft Structural Analysis, 3rd edition, Oxford, United Kingdom: Butterworth-Heinemann, 2018.
-
G. Moon and E. Lee, “Combined effects of optimized heat treatment and nickel coating for the improvement of interfacial bonding in aluminum–iron alloys hybrid structures,” Applied Sciences, vol. 11, no, 4, p. 1501, 2021.
[https://doi.org/10.3390/app11041501]

-
M. Wang, D. H. Xiao, and W. S. Liu, “Effects of Si addition on microstructure and properties of magnesium alloys with high Al and Zn contents,” Vacuum, vol. 141, pp. 144-151, 2017.
[https://doi.org/10.1016/j.vacuum.2017.04.005]

- S. O. Adeosun, S. A. Balogun, L. O. Osoba, W. A. Ayoola, and A. M. Oladoye, “Effects of Cu and Zn addition on the mechanical properties of structural aluminum alloy,” Manufacturing Technology, vol. 3, pp. 103-110, 2011.
- N. Yass and K. Nagham, “Study the effects of magnesium addition on microstructure, electrical conductivity and some mechanical properties of pure aluminum,” Journal of Babylon University, Engineering Sciences, vol. 25, no. 1, pp. 251-260, 2017.
-
H. R. Ammar, A. M. Samuel, and F. H. Samuel, “Porosity and the fatigue behavior of hypoeutectic and hypereutectic aluminum–silicon casting alloys,” International Journal of Fatigue, vol. 30, no. 6, pp. 1024-1035, 2008.
[https://doi.org/10.1016/j.ijfatigue.2007.08.012]

-
L. Lu and A. K. Dahle, “Iron-rich intermetallic phases and their role in casting defect formation in hypoeutectic Al-Si alloys,” Metallurgical and Materials Transactions A, vol. 36, pp. 819-835, 2005.
[https://doi.org/10.1007/s11661-005-0196-y]

-
J. A. Taylor, “Iron-containing intermetallic phases in Al-Si based casting alloys,” Procedia Materials Science, vol. 1, pp. 19-33, 2012.
[https://doi.org/10.1016/j.mspro.2012.06.004]

-
Q. G. Wang, D. Apelian, and D. A. Lados, “Fatigue behavior of A356-T6 aluminum cast alloys. Part I. Effects of casting defects,” Journal of Light Metals, vol. 1, no. 1, pp. 73-84, 2001.
[https://doi.org/10.1016/S1471-5317(00)00008-0]

-
C. H. Cáceres and B. I. Selling, “Casting defects and the tensile properties of an AlSiMg alloy,” Materials Science and Engineering: A, vol. 220, no. 1-2, pp. 109-116, 1996.
[https://doi.org/10.1016/S0921-5093(96)10433-0]

-
B. Li, Y. Shen, and W. Hu, “Casting defects induced fatigue damage in aircraft frames of ZL205A aluminum alloy – A failure analysis,” Materials & Design, vol. 32, no. 5, pp. 2570-2582, 2011.
[https://doi.org/10.1016/j.matdes.2011.01.039]

- J. R. Davis, Aluminum and Aluminum Alloys, ASM International, United States of America, 2001.
-
J. W. Gao, D. Shu, J. Wang, and B. D. Sun, “Effects of Na2B4O7 on the elimination of iron from aluminum melt,” Scripta Materialia, vol. 57, no. 3, pp. 197-200, 2007.
[https://doi.org/10.1016/j.scriptamat.2007.04.009]

-
J. W. Gao, D. Shu, J. Wang, and B. D. Sun, “Study on iron purification from aluminium melt by Na2B4O7 flux,” Materials Science and Technology, vol. 25, no. 5, pp. 619-624, 2009.
[https://doi.org/10.1179/174328408X339288]

-
M. A. Moustafa, “Effects of iron content on the formation of β-Al5FeSi and porosity in Al–Si eutectic alloys,” Journal of Materials Processing Technology, vol. 209, no. 1, pp. 605-610, 2009.
[https://doi.org/10.1016/j.jmatprotec.2008.02.073]

-
N. Vanderesse, É. Maire, A. Chabod, and J. -Y. Buffière, “Microtomographic study and finite element analysis of the porosity harmfulness in a cast aluminium alloy,” International Journal of Fatigue, vol. 33, no. 12, pp. 1514-1525, 2011.
[https://doi.org/10.1016/j.ijfatigue.2011.06.010]

-
J. Lee, Y. Jeong, D. Shim, and E. Lee, “Microstructural evolution and martensitic transformation in FeCrV alloy fabricated via additive manufacturing,” Materials Science and Engineering: A, vol. 809, 140943, 2021.
[https://doi.org/10.1016/j.msea.2021.140943]

- J. E. Gruzleski and B. M. Closset, “The treatment of liquid aluminum–silicon alloys,” American Foundrymen’s Society, Des Plaines, 1990.
-
G. S. Cho and K. H. Choe, “Study on the rationalization of aluminium casting alloys for automobiles components,” Journal of the Korea Foundry Society, vol. 31, no. 6, pp. 319-325, 2011 (in Korean).
[https://doi.org/10.7777/jkfs.2011.31.6.319]

-
I. Jo, C. Ahn, and E. Lee, “High-temperature tensile deformation behavior and failure mechanisms of Al-10Si-Mn-Mg high-pressure die-cast alloy,” Journal of Advanced Marine Engineering and Technology, vol. 43, no. 10, pp. 788-792, 2019.
[https://doi.org/10.5916/jkosme.2019.43.10.788]

-
H. S. Chul and S. Y. Kwak, “Effects of shrinkage defect on fracture impact energy of A356 cast aluminum alloy,” Journal of Korea Foundry Society, vol. 34, no. 1, pp. 22-26, 2014 (in Korean).
[https://doi.org/10.7777/jkfs.2014.34.1.022]

-
J. Lazaro-Nebreda, J. B. Patel, and Z. Fan, “Improved degassing efficiency and mechanical properties of A356 aluminium alloy castings by high shear melt conditioning (HSMC) technology,” Journal of Materials Processing Technology, vol. 294, 117146, 2021.
[https://doi.org/10.1016/j.jmatprotec.2021.117146]

-
M. Wang, W. Xu, and Q. Han, “Effects of heat treatment on controlling the morphology of AlFeSi Phase in A380 alloy,” International Journal of Metalcasting, vol. 10, no. 4, pp. 516-523, 2016.
[https://doi.org/10.1007/s40962-016-0068-9]

-
J. G. Conley, J. Huang, J. Asada, and K. Akiba, “Modeling the effects of cooling rate, hydrogen content, grain refiner and modifier on microporosity formation in Al A356 alloys,” Materials Science and Engineering: A, vol. 285, no. 1-2, pp. 49-55, 2000.
[https://doi.org/10.1016/S0921-5093(00)00665-1]

- J. Wisniak, “Borax, boric acid, and boron - From exotic to commodity,” Indian Journal of Chemical Technology, vol. 12, no. 4, pp. 488-500, 2005.
-
G. A. Mengesha, J. P. Chu, B. -S. Lou, and J. -W. Lee, “Effects of processing parameters on the corrosion performance of plasma electrolytic oxidation grown oxide on commercially pure aluminum,” Metals, vol. 10, no. 3, p. 394, 2020.
[https://doi.org/10.3390/met10030394]

-
L. Qiyang, L. Qingchun, and L. Qifu, “Modification of Al-Si alloys with sodium,” Acta Metallurgica et Materialia, vol. 39, no. 11, pp. 2497-2502, 1991.
[https://doi.org/10.1016/0956-7151(91)90064-8]

-
H. D. Alamdari, D. Dubé, and P. Tessier, “Behavior of boron in molten aluminum and its grain refinement mechanism,” Metallurgical and Materials Transactions A, vol. 44, pp. 388-394, 2013.
[https://doi.org/10.1007/s11661-012-1388-x]

- B. H. Kim, J. K. Kim, and S. M. Lee, “Magnetic separation of Fe contaminated Al-Si cutting chip scraps and evaluation of solidification characteristics,” Journal of the Korean Foundrymen’s Society, vol. 29, no. 1, pp. 38-44, 2009 (in Korean).
-
J. Fu, S. Wang, and K. Wang, “Influencing factors of the coarsening behaviors for 7075 aluminum alloy in the semi-solid state,” Journal of Materials Science, vol. 53, pp. 9790-9805, 2018.
[https://doi.org/10.1007/s10853-018-2246-z]

- M. H. Yoo, K. W. Kim, and M. S. Moon, “A study on mechanical properties according to changes in microstructure and precipitation phase of A356 alloy and the Mn and the St added to A356 alloy,” Journal of the Korean Society of Mechanical Technology, vol. 22, no. 4, pp. 740-746, 2020 (in Korean).
-
S. G. Irizalp and N. Saklakoglu, “Effect of Fe-rich intermetallics on the microstructure and mechanical properties of thixoformed A380 aluminum alloy,” Engineering Science and Technology, an International Journal, vol. 17, no. 2, pp. 58-62, 2014.
[https://doi.org/10.1016/j.jestch.2014.03.006]

-
Y. Zedan, F. H. Samuel, A. M. Samuel, and H. W. Doty, “Effects of Fe intermetallics on the machinability of heat-treated Al-(7-11) % Si alloys,” Journal of Materials Processing Technology, vol. 210, no. 2, pp. 245-257, 2010.
[https://doi.org/10.1016/j.jmatprotec.2009.09.007]

-
C. Ahn, I. Jo, C. Ji, S. Cho, B. Mishra, and E. Lee, “Creep behavior of high-pressure die-cast AlSi10MnMg aluminum alloy,” Materials Characterization, vol. 167, 110495, 2020.
[https://doi.org/10.1016/j.matchar.2020.110495]