Improvement of throwing power of cathodic protection in reinforced concrete structure with conductive mortar
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Cathodic protection for the rehabilitation of reinforced concrete structures prohibits rebar corrosion, such as Impressed Current Cathodic Protection (ICCP) systems and Sacrificial Anode Cathodic Protection (SACP) systems. In the case of the SACP system, it is difficult to protect against rebar corrosion with cathodic protection because of the high resistivity of the concrete, and the concrete overlays should be sufficiently conductive to pass the cathodic protection current from the anode to the rebar because the throwing power of the cathodic protection provided by the sacrificial anode is limited by the high resistivity of the concrete. Therefore, it is necessary to study the properties of conductive mortar for the effective cathodic protection of reinforced concrete. In an evaluation of mortar containing a conductive material as an electrolyte for a concrete overlay to improve the cathodic protection performance, an admixture suitable for concrete showed the lowest resistivity value, and it can be used as an admixture for conductive mortar.
Keywords:
Cathodic protection, Rehabilitation, Resistivity, Admixture, Concrete1. Introduction
Reinforced concrete has become the most common material for structures over the last few years with the use of composite materials, and is one of the most popular materials for building construction. In recent years, convergence and hybrid technologies in various fields have been introduced, and various attempts have been made to develop concrete materials with the development of science and technology.
However, despite the development of materials and mixed design technology to suppress cracks in reinforced concrete, the technology to date cannot prevent cracks from occurring in reinforced concrete. It is inevitable that in areas where there is a corrosive environment, cracks are initiated and propagated in a reinforced concrete structure. Therefore, even if a crack occurs, research is needed to develop a method that suppresses the rebar corrosion caused by deterioration factors in the cracked area [1].
Over the past years, there has been an increase in the application of cathodic protection for the rehabilitation of reinforced concrete structures to prohibit rebar corrosion, such as Impressed Current Cathodic Protection (ICCP) systems and Sacrificial Anode Cathodic Protection (SACP) systems. It is difficult to protect against rebar corrosion with cathodic protection because of the high resistivity of the concrete, especially using the SACP system, and a concrete overlay should be sufficiently conductive to pass the cathodic protection current from the anode to the rebar [2].
In this study, a method for improving the cathodic protection of reinforced concrete was confirmed by applying mortars containing various conductive materials as electrolytes as a method of suppressing the corrosion of reinforced concrete with the SACP system.
2. Cathodic protection systems for the reinforced concrete
It has strong alkalinity due to the calcium hydroxide generated by the hydration of cement, and forms a protective film called a passive layer on the surface of the reinforcement; thus, the reinforcement in the concrete is not significantly corroded.
As a result, concrete has been recognized as an almost semi-permanent material, but the deterioration of reinforced concrete structures is increasing as a result of salt damage, carbonation, etc. In particular, in the case of port structures or bridge structures adjacent to the coast, rebar corrosion due to salt damage is serious.
The dissolved chloride ions inside the concrete play a major role in the corrosion damage of concrete, and increase the active corrosion rate of the rebar by damaging the passive film of the rebar [3].
Much effort has been made to improve the durability of reinforced concrete in a salty environment, and typical methods include high-density concrete, increased concrete cover, concrete surface coating, reinforced epoxy coating, and cathodic protection. These can extend the durability and life of a product.
Among them, the cathodic protection system was introduced as a corrosion protection method for underground and seawater metals, and is currently widely used as a corrosion protection method for underground buried pipes, ships, and port steel structures.
In the 1970s, it began to be applied to reinforced concrete structures; in the 1990s, many practical applications were made internationally, and the effectiveness of rebar corrosion prevention has been widely proven. It has been gradually applied to port structures in Korea, and the scope of its application is now expanding to bridge structures [4].
The cathodic protection method is not a defensive method that blocks corrosion factors from the environment or delays corrosion for a certain period of time, but an aggressive method that actively copes by supplying cathodic protection current (electrons) to the reinforcing bar.
The cathodic protection methods are divided into two types according to the method used to supply the protection current. The Impressed Current Cathodic Protection (ICCP) forcibly supplies protection current from an external DC power supply unit. The Sacrificial Anode Cathodic Protection (SACP) method connects the reinforcement to a sacrificial anode made of a metal that is more corrosive than the protected metal (reinforced), which protects the reinforcement as a result of the potential difference between the two metals. Because the ICCP system can control the supply current, even if the resistivity of concrete is high, it is not a significant problem. However, in the SACP system, sufficient protection current cannot be supplied from the anode to the cathode because of the lack of throwing power of the SACP system, resulting in lower protection efficiency, and the reach of the protection current is extremely limited [5].
Therefore, it is necessary to solve the underprotection problem of the SACP system when applied to reinforced concrete and to study the characteristics of conductive mortar for effective cathodic protection. To improve the supply of protection current between the anode and cathode (reinforcing bar), the efficiency of the cathodic protection is improved by lowering the resistivity of concrete using a conductive mortar.
In the case of the SACP system, the range of use is extremely limited in environments with high resistivity, such as reinforced concrete. To compensate for these shortcomings, a conductive mortar can be applied to the splash zone or atmospheric zone by expanding its use from the area of the tidal zone or submerged zone. By applying it to the SACP system, cathodic protection of the entire structure is possible by expanding the corrosion protection range to a region with high resistivity, that is, the underprotection area.
3. Conductive admixture for cement mortar
Conductive mortar is made by adding mixed materials and admixtures to impart conductivity to the general cement mortar. The purpose of the mixed material is to make a conductive mortar using a material that can contain moisture in order to lower the specific resistance of the mortar [6].
The types of materials used to make a conductive mortar containing moisture and reduce the resistivity are shown in Figure 1. These include zeolite, activated carbon, activated alumina, bentonite, perlite, and geopolymer. Among these, activated carbon, zeolite, silica gel, and activated alumina are commercially used as general moisture adsorbents, and activated carbon is used as a multipurpose adsorbent. Silica gel and activated alumina can be used in various forms for special purification; however, they are mainly used as drying agents. In addition, zeolite is mostly used as an ion exchange or catalyst owing to its unique surface chemistry and structural characteristics of crystal pores.
John P. Broomfield insisted that it is the transfer of charge by the diffusion of ions, not the transfer of electrons that imparts conductivity, and argued that carbon or metallic materials are not suitable as conductive materials used as fillers [7].
Christopher L Page approached it in terms of pH, and insisted that conductivity is also important in the SACP method, but that a passivation film is not formed on the anode surface while the cathodic protection current flows. In addition, he suggested that among the anodes used as sacrificial anodes in concrete, zinc is limited to corrosion at pH 13.3 or higher, and is most suitable at pH 14 or higher. To adjust the pH of the mortar to a value greater than 14, a catalyst such as sodium hydroxide, calcium hydroxide, or lithium hydroxide must be mixed with water [8].
All of the previous studies have focused on earth resistance-reducing materials, electromagnetic shielding concrete, antistatic concrete, intelligent concrete, and heating concrete related to conductive concrete or mortar, with no studies on conductive mortar for sacrificial anodes.
4. Experimental method
Portland cement and standard sand are usually used to make conductive mortar. For the materials to be mixed to impart conductivity, the relative specific gravity must be measured compared to standard sand because the amount of mixed materials needed to obtain the optimum mixing ratio should be calculated considering the difference in the conductivity of each material.
The mixing ratio of cement and sand is usually 1:2, and the amount of admixture is 30% based on the amount of sand. The mixing design is presented in Table 1. At this time, the amount of mixing water to be used could be determined according to the characteristics of the mixed material through a flow test, as shown in Figure 2.
To measure the resistivity of the mortar specimen, the 4-pin method proposed by Wenner was applied, which is the most common method for measuring the resistivity of concrete. The distance between each of the four electrodes was fixed at 1 cm, and they were arranged in one row. After the mortar specimens were prepared and cured for a certain period, the concrete resistivity was measured over time using a resistivity meter (Nilsson model 400 soil resistivity meter), as shown in Figure 3. The resistivity of the mortar specimens was measured for 90 days at a constant temperature of 23 °C.
5. Experimental results
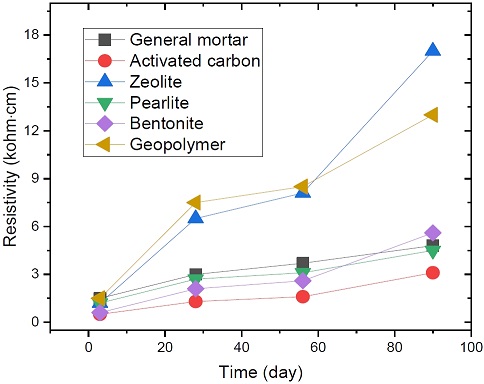
The resistivity measurement results for the mortar specimens are shown in the Figure 4.
First, in the case of activated alumina, it was excluded from the resistivity measurement because of the obvious change in appearance. It was judged that it was not suitable as a mixed material because cracks were generated on the surface of the mortar as part of this change in appearance.
The measured resistivity values for all the mortar specimens showed a tendency to gradually increase over time.
The resistivity of the mortar mixed with activated carbon was lower than that of the general mortar. It increased rapidly after 56 days. It was judged that the evaporation of moisture inside the activated carbon with large pores was accelerated because the dry state continued with no water supplied as time passed.
Activated carbon was considered to be the most suitable material for a conductive mortar because it had a remarkably low resistivity value compared to the other conductive mortar materials, and the resistivity value did not increase rapidly over time but remained at a constant value. In addition, the activated carbon did not significantly change its shape after mortar curing. It is believed that it can be commercialized through various additional experiments in the future, which, for example, should make it possible to find the most stable value as a result of mixed material tests.
Zeolite is known as the most suitable material among the various conductive mortar materials, but it was judged to be unsuitable as an electrolyte for cathodic protection systems because it had a higher resistivity value than the general mortar. To use zeolite as an electrolyte material for a conductive mortar, it would be necessary to reduce its resistivity using other methods such as manufacturing zeolite particles of various sizes.
Pearlite exhibited characteristics similar to those of activated carbon and maintained a good resistivity value, but it is expected that the strength of the mortar will be poor because the strength of pearlite itself is weak, and it was judged to be unsuitable for esthetic use as an admixture because of the occurrence of cracks on the surface.
Bentonite maintained a good initial resistivity value, but it is not suitable for use as a conductive mortar because its resistivity value increased rapidly after 56 days. However, it is considered to be highly useful in environments where dry and humid conditions are repeated, such as tidal zones in marine environments.
The geopolymer was not suitable as a conductive mortar material because it exhibited a high resistivity value due to the characteristics of the mortar. However, it was considered a good mixture material for improving the durability.
6. Conclusion
As a result of evaluating mortars containing conductive materials as electrolytes for concrete overlays to improve the cathodic protection performance, the following conclusions were obtained.
- 1. Activated alumina was excluded from the materials for the conductive mortar because of the obvious changes in appearance. It was not suitable as an admixture because cracks were generated on the surface of the mortar as part of this change in appearance.
- 2. Pearlite exhibited characteristics similar to those of activated carbon and maintained a good resistivity value, but it is expected that the strength of the mortar will be poor because the strength of pearlite itself is weak.
- 3. Among the admixtures used in the experiment, the mortar specimen mixed with activated carbon showed the lowest resistivity value, and it is believed that it could be used as an admixture for conductive mortar.
- 4. It was judged that it will be possible to solve the problem of using the SACP system for reinforced concrete structures by applying a conductive mortar.
Author Contributions
Conceptualization, J. M. Ha and J. A. Jeong; Methodology, J. M. Ha; Software, J. M. Ha; Validation, J. M. Ha and J. A. Jeong; Formal Analysis, J. M. Ha; Investigation, J. M. Ha; Resources, J. M. Ha; Data Curation, J. M. Ha; Writing—Original Draft Preparation, J. M. Ha; Writing—Review & Editing, J. A. Jeong; Visualization, J. M. Ha; Supervision, J. A. Jeong; Project Administration, J. A. Jeong; Funding Acquisition, J. A. Jeong.
References
-
L. Bertolini and E. Redaelli, “Throwing power of cathodic prevention applied by means of sacrificial anode to partially submerged marine reinforced concrete piles: Results of numerical simulations,” Corrosion Science, vol. 51, no. 9, pp. 2218-2230, 2009.
[https://doi.org/10.1016/j.corsci.2009.06.012]

-
F. Lollini, E. Redaelli, and L. Bertolini, “Analysis of the parameters affecting probabilistic predictions of initiation time for carbonation-induced corrosion of reinforced concrete structures,” Materials and Corrosion, vol. 63, no. 12, pp. 1059-1068, 2012.
[https://doi.org/10.1002/maco.201206720]

-
P. Schiessl and M. Raupach, “Laboratory studies and calculations on the influence of crack width on chloride-induced corrosion of steel in concrete,” Materials Journal, vol. 94, no. 1, pp. 56-61, 1997.
[https://doi.org/10.14359/285]

- D. A. Jones, Principles and Prevention of Corrosion, 2nd Edition, Upper Saddle River, New Jersey, the U.S.: Pearson, 1995.
-
J. A. Jeong, C. K. Jin, and W. -S. Chung, “Tidal water effect on the hybrid cathodic protection systems for marine concrete structures,” Journal of Advanced Concrete Technology, vol. 10, no. 12, pp. 389-394, 2012.
[https://doi.org/10.3151/jact.10.389]

-
X. Jing and Y. Wu, “Electrochemical studies on the performance of conductive overlay material in cathodic protection of reinforced concrete,” Construction and Building Materials, vol. 25, no. 5, pp. 2655-2662, 2011.
[https://doi.org/10.1016/j.conbuildmat.2010.12.015]

-
J. Broomfield, Corrosion of Steel in Concrete, 2nd edition: Taylor & Francis, 2003.
[https://doi.org/10.1201/9781482265491]

- C. L. Page, “Repair of corroded reinforcement in concrete using sacrificial anodes,” United States, No. 6,022,469, Feb. 8, 2000.