Experimental study on the characteristics of phase change materials as a cold storage method for refrigerant air dryers
Copyright ⓒ The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study examined the fundamental characteristics of phase change materials (PCMs) for the development of a novel refrigerant air dryer. The PCMs were used as a cold storage method in order to overcome the drawbacks associated with conventional energy-saving refrigerant air dryers. For this purpose, an experiment was performed on two types of PCMs with a test device capable of simulating the interior of a heat exchanger. During this study, it was found that both PCMs exhibited good performance and suitable properties, given that the temperature and volume changes were small during phase change, and that PCMs contain dissolved gas. PCM A exhibited superior performance as a cold storage method than PCM B when PCM A was completely frozen and proceeded to melt under general conditions. However, under the actual operating conditions of the air dryer, PCM B exhibited superior performance according to the operating temperature owing to the partial changes in phases of both PCMs.
Keywords:
Air dryer, Cold storage system, Energy savings, Phase change material1. Introduction
Compressed air is widely used throughout the industry in controls, processing, and other miscellaneous operations. However, moisture contained in the air, captured during the compression process, leads to the corrosion and malfunction of equipment and causes extensive damage to products during the manufacturing process. Thus, it is essential to install air dryers in order to remove moisture from air compression systems. Air dryers are primarily divided into refrigerating and adsorbing types based on the moisture removal method. Refrigerant air dryers are commonly used unless compressed air with a very low dew point is required. Refrigerant air dryers frequently start and stop, based on the load characteristics of the compressed air, and thus, are disadvantageous in terms of energy efficiency as the system operation generally maintains a continuous operating condition for the safety of the compressor in the refrigeration system. To overcome this shortcoming, in place of the refrigerant air dryer, in which the refrigerant directly refrigerates and desiccates the compressed air within the same heat exchanger via direct refrigeration, a cold storage refrigerant air dryer with an indirect refrigeration system was developed.
The refrigerant in the brine chiller initially refrigerates the brine (thermal mass), which is a cold storage medium, to a predetermined temperature, and this brine circulates the compressed air chiller to refrigerate the compressed air for the second time. The cold storage refrigerant air dryer with indirect refrigeration has two separate chillers, which allows placing a sufficiently large brine storage compartment for On/Off control of the chiller that significantly increases the energy efficiency of the air dryer. Nevertheless, the indirect cold storage refrigerant air dryer was not widely adopted. This was because the system was somewhat complicated as it required a separate large capacity storage tank and auxiliary equipment, and there were disadvantages such as large installation area and high costs. This study introduces a novel approach of direct refrigeration phase change material (PCM)-type refrigerant air dryer that can inherit the advantages and overcome the disadvantages of the direct refrigerant air dryer and indirect cold storage refrigerant air dryers systems [1]-[5]. While conventional indirect cold storage refrigerant air dryers use sensible heat from brine, direct refrigeration PCM-type refrigerant air dryers use PCM, which mainly utilizes latent heat, as the cold storage medium to overcome the insufficient cold storage space. This new method has a simple structure and requires a small installation area as the PCMs are frozen by the refrigerant, in the same heat exchanger. Furthermore, the moisture in the compressed air utilizes the latent heat when the PCM melts and also exhibits high energy efficiency due to the possible On/Off control of the chiller. In this work, to select the appropriate cold storage medium for a direct PCM-type refrigerant air dryer, experiments were conducted to study the physical and thermal properties of PCMs that showed potential as a cold storage medium.
2. Method
2.1 Experimental Device
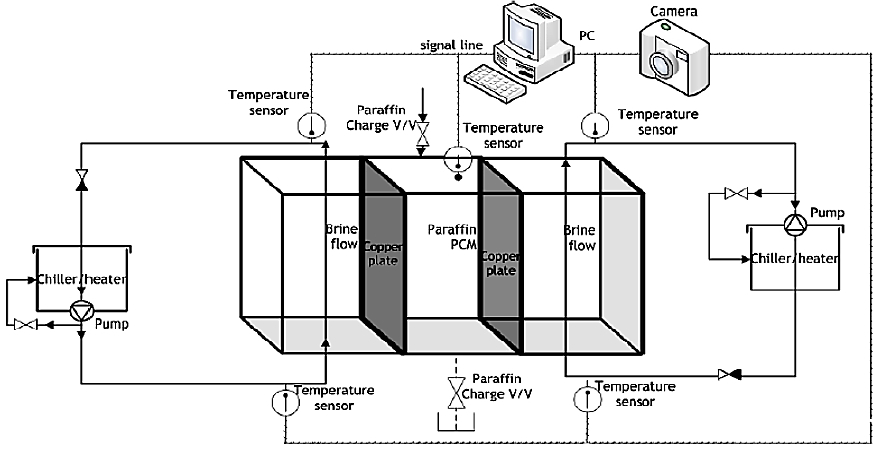
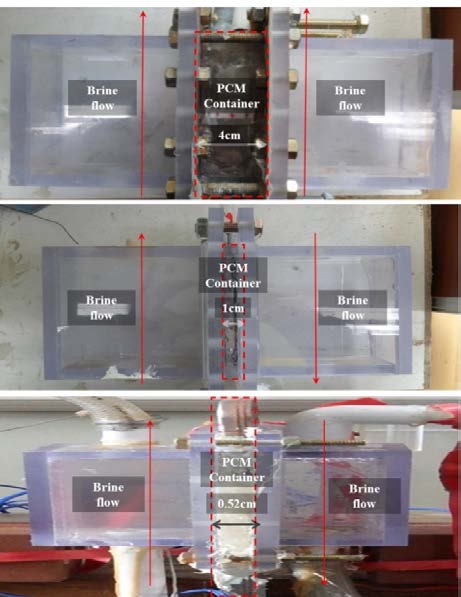
Figure 1 is a schematic of the test device used to investigate the physical and thermal properties associated with the freezing and melting of the PCMs selected in this study. The actual heat exchanger is configured such that heat transfer from the refrigerant, cold storage medium, and compressed air occurs simultaneously within the same heat exchanger. The box inside the dotted lines at the center of the figure is the test chamber that contains the PCM, and brine cooled at a predetermined temperature and separated by thin copper plates circulates the PCM. Experiments are conducted by varying the width of the PCM cool storage container as 4 cm, 1 cm, and 0.52 cm, as shown in Figure 2.
The 4-cm-wide PCM cold storage container is primarily used for observing the phenomena during the freezing and melting of the PCM. The 1-cm-wide cold storage container is used for thermal studies, while the cold storage container of width 0.52 cm, which is the width of the actual developed product, is used in performance comparison studies. Temperature data relating to the interior of the PCM cold storage container are collected by placing the temperature sensors in a way that prevents any effect on the physical phenomena. In addition, the experimental progress is recorded as images using conventional and thermal imaging cameras.
While detailed formulations of the PCMs used in this study cannot be disclosed due to confidentiality agreement with the industry collaborator, these materials are paraffin-based compounds with freezing points modified to 6.5 ℃ and 3.0 ℃. For the sake of convenience, PCM A refers to the material with a freezing point of 6.5 ℃, and PCM B refers to that of 3.0 ℃.
2.2 Experimental Details and Methods
To collect information such as the shape, frozen state, and change in volume of PCM A and B during freezing and melting, the 4-cm-wide cold storage container was used to consider the start of the experiment as the moment when the brine, maintained at a predetermined temperature, began to circulate. Throughout the experimental process, the shape and state of the PCMs were photographed and observed. At the same time, the temperature was recorded, and the change in volume was measured from the change in liquid level of the sight glass tube installed on the top of the cold storage container. Brine flowed from the bottom to the top, and the temperature was maintained at –10 ℃ during freezing and 10 ℃ during melting. Additionally, the cold storage medium was placed in a beaker and frozen in a freezer to observe the state of the surface and the internal crystal structure. Also, to investigate the growth of the frozen layer thickness in PCM A and B, the 1 cm-wide cold storage container was used wherein only the left side was exposed to the brine circulating at –10 ℃. During this process, the right side was in contact with stagnant air to suppress heat transfer to the maximum possible extent. To examine the performance characteristics of PCMs A and B in the plate heat exchanger (actual developed product), the left and right sides of the 0.52-cm-wide cold storage container were repeatedly used as brine compartments during freezing and melting, respectively, to analyze the suitability of the PCMs. Here, the circulating brine for freezing was experimented with using four temperature conditions of –6 ℃, -3 ℃, 0 ℃, and 3 ℃, and the brine for melting was at 10 ℃, both flowing countercurrent to each other.
3. Results
3.1 Physical Properties of PCMs A and B during Phase Transition
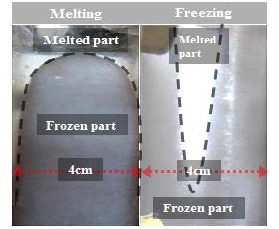
Figure 3 shows a photo of the appearance of PCM A undergoing phase change in the 4-cm-wide cold storage container, where the left side is under the melting state, and the right side is under the freezing state. The case of PCM B exhibited an identical tendency to that of PCM A. The figure on the left shows that the frozen layer is broader in the lower part of the PCM upon freezing. This is interpreted as the effect of weak natural convection in the liquid layer. In the figure on the right, the shape of the upper frozen layer is thicker and becomes drastically thinner in the lower part of the PCM upon melting. This is attributed to the strong natural convection in the melted layer that collides with the upper frozen layer that rises to the top along both heat transfer surfaces. In addition, it was observed from freezing tests that the interface of the frozen layer grew in the form of tiny needles. Moreover, to identify the internal state of the frozen layer, each of the cold storage medium contained in a beaker was frozen in a freezer at –3.0 ℃, and its internal frozen layer was observed as shown in Figure 4. According to the observation results in Figure 4, the frozen layer of PCM A was rigid and opaque with thin crystals. At the same time, PCM B was soft and translucent and comprised of relatively larger crystals. In particular, PCM B consisted of an inhomogeneous frozen layer that was filled with a considerable amount of liquid between the frozen crystals. In contrast, the frozen layer of PCM A exhibited a mild inhomogeneous state. Such an inhomogeneous frozen layer was considered to result from the incongruent freezing point of the cold storage medium as a compound. However, there was no change in its fundamental properties as a cold storage medium even after repeated use while undergoing freezing and melting.
Figure 5 displays the difference in liquid level of the sight glass tube installed on top of the cold storage container during freezing and melting of PCM A and B. It can be seen that the volumes of PCMs A and B contracted upon freezing and expanded upon melting. As a 1-cm change in the liquid level here corresponds to an approximate 1% difference in volume, an approximately 14% change in volume occurred in PCM A and 15% in PCM B upon freezing. Therefore, as a significant change in volume accompanied PCM A and B, such characteristics must be taken into account for the design of a cold storage container and refill of the cold storage medium.
The image above in Figure 6 shows the presence of a dissolved gas that exists inside the PCM frozen layer. The image below displays the liquid level before and after removal of the dissolved gas, showing that approximately 2% of the gas was present inside the initial cold storage medium. The existence of such dissolved gas indicates a reduction in the charge amount of the cold storage medium and should be removed before refilling. For the removal of the dissolved gas, separating the gas via repeated freezing and melting is possible but complicated. Thus, the cold storage medium heating method is recommended as it is the most convenient and requires a short amount of time. This method involves heating the exterior at 80 ℃ for approximately 1 hour prior to refilling the cold storage medium. Most of the gas was removed by heating the cold storage medium a single time only. The reason for heating the medium at 80 ℃ is because the ignition point of the PCM is near 95 ℃.
3.2 Thermal Properties of PCMs A and B
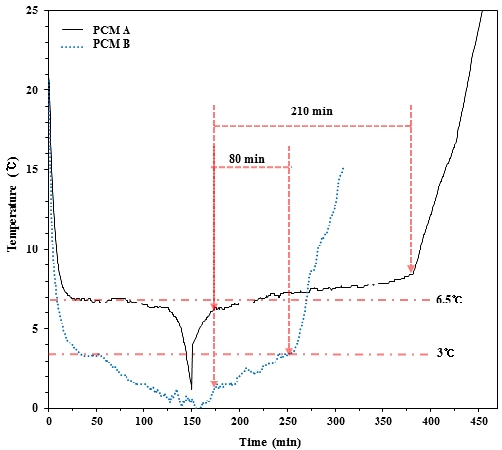
Figure 7 shows the change in temperature in relation to freezing and melting of PCM A and B in the 4-cm-wide cold storage container. The temperature of the brine is at –10 ℃ and 10 ℃ during freezing and melting, respectively, and the temperature is measured at the center of the cold storage container. The figure demonstrates that cold storage from sensible heat proceeds for a relatively short amount of time due to the rapid temperature drop that initially occurs with the brine circulation for freezing. Afterward, the change is gradual, and a steady temperature is maintained, reflecting the cold storage process from latent heat. PCM A more clearly demonstrates this process. Following a relatively long latent heat storage process, a sensible heat storage process in the solid state occurs and results in a rapid drop in temperature. When the temperature at the center of the cold storage container reaches 0 ℃, brine at 10 ℃ for melting is circulated, and the mode switches to a melting operation, resulting in a rapid temperature increase from the release of cold latent heat in the cold storage medium. Then, an interval with a nearly constant temperature appears, which corresponds to the dissipation of cold latent heat during the solid-to-liquid transition. This interval disappears after about 210 min for PCM A and 80 min for PCM B, and the temperature of the cold storage medium sharply rises as it enters the cold latent heat dissipation interval of the liquid. In the figure, the cold latent heat dissipation interval of PCM A is longer than that of PCM B due to the difference in the amount of transferred heat from the temperature difference between the brine for melting and the cold storage medium. However, because the frozen layer of PCM B consists of an inhomogeneous layer with coexisting liquid and solid states as mentioned in Section 3.1.1., this is considered to be attributed to the fact that the bulk of the frozen layer is not entirely being used for latent heat storage and thus leads to a lower cold storage capability than that of PCM A. Furthermore, the temperature of PCM A for latent heat cold storage and cold release distinctly occurs near the freezing point, but this is rather obscure in PCM B. As noted in Section 3.1.1., this may be due to the formation of an inhomogeneous frozen layer that results from the incongruent freezing point of the cold storage medium as a compound.
Looking at the freezing and melting points in greater detail, the freezing and melting of PCM A occurred at similar temperatures at which the freezing point was 6–7 ℃ and the melting point was 6–8 ℃, and the change in temperature within the phase transition interval was as low as 2 ℃. Also, PCM B froze and melted at similar temperatures at which the freezing point was 3–4℃, and the melting point was 1–3 ℃. The temperature difference within the phase transition interval was as low as 2 ℃. Thus, it can be concluded that the two PCMs exhibit appropriate characteristics that maintain a constant PCM temperature for compressed air moisture removal in the evaporator of a PCM dryer.
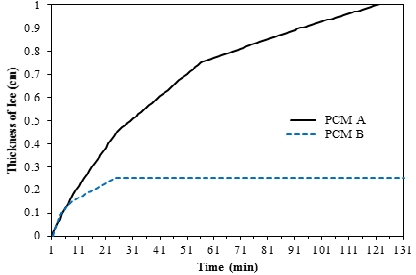
Figure 8 presents a graph of the thickness of ice in the PCM with respect to freezing time during freezing in a cold storage container with brine circulated at –10 ℃. PCM A shows that the increase in the thickness of ice slows down quickly as freezing time continues. PCM B also exhibits similar behavior, and although the initial ice thicknesses were comparable, it can be seen that the increase in ice thickness in PCM B slows at an increased rate. In addition, almost no freezing occurred after the ice thickness of PCM B reached 0.25 cm. During the operation of a PCM dryer, a refrigerant compressor is run to supply the refrigerant for cooling the PCM and is stopped once the PCM is frozen; here, a fast freezing speed of the PCM is crucial during the supply of refrigerant because the efficiency becomes higher when the refrigerant compressor operates for a shorter amount of time. Therefore, the experimental results demonstrate that PCM A shows more favorable features as a cold storage medium than PCM B, as it freezes more quickly and exhibits a larger final ice thickness.
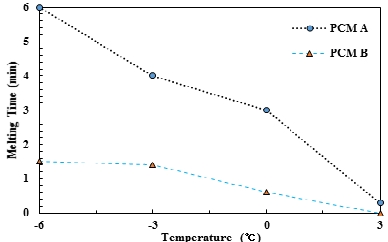
Figure 9 displays the time it takes to completely melt a frozen PCM using brine at 10 ℃ after fully freezing the PCM under four temperature conditions of -6 ℃, -3 ℃, 0 ℃ and 3 ℃ in the 0.52-cm-wide cold storage container (the actual version of the heat exchanger). PCM A and PCM B both demonstrate a shorter time for complete melting with higher freezing temperatures. This can also be explained from Figure 7, where a long latent heat interval appears at 6.5 ℃ when PCM A is cooled to 3 ℃, and a short sensible heat interval appears from 6.5 ℃ to 3 ℃. If it is cooled to –6 ℃, following the latent heat interval, the sensible heat interval where cooling occurs from 6.5 ℃ to –6 ℃ will be longer. Thus, a greater overall cold storage can be achieved with lower freezing temperature, owing to the increase in the cold storage effect of sensible heat between latent heat and sensible heat. Similarly, PCM B is capable of enhanced cold storage at low temperatures. The melting time is longer at low temperatures than at high temperatures because the resulting melting time is prolonged with increased cold storage at low temperatures.
3.3 PCM Cold Storage Performance Characteristics under PCM Dryer Operating Conditions
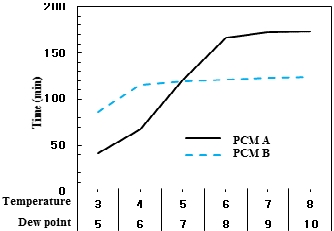
Figure 10 presents the melting time under six temperature conditions where the PCM, frozen with brine at -10 ℃ in the 4-cm-wide cold storage container, is melted with brine at 10 ℃ until the PCM temperature reaches 3 ℃, 4 ℃, 5 ℃, 6 ℃, 7 ℃, and 8 ℃. This is an experimental condition that takes into account the operating conditions of the PCM dryer explored in this study being set to temperatures between 3 ℃ and 8 ℃. According to the results, PCM A showed a higher cold storage performance than PCM B when the melting temperature condition was above 5 ℃. It was found that the cold storage performance of PCM B was better at temperatures below 5 ℃. This is because the advantage of PCM A in cold storage—demonstrated in the experimental results in Section 3.1.2 that show the benefits of PCM A for cold storage as it exhibits homogeneous phase transition characteristics below the freezing point—disappears because the PCM does not undergo complete phase transition inside the evaporator of an actual PCM dryer during operation. In addition, the reason for the improvement in the cold storage performance of PCM B below the melting temperature range of 5 ℃ is attributed to the characteristics from the difference in freezing points. Based on the actual PCM dryer, assuming that the PCM temperature setting configures the compressor to start at 5 ℃ and stop at 3 ℃, PCM A always exists as a solid because its freezing point is 6.5 ℃ and cannot utilize the latent heat from phase transition. On the other hand, PCM B undergoes phase change at 3–4 ℃ and can use the latent heat, resulting in PCM B being more desirable for use in the product.
However, taking a closer look at the operating condition of the actual PCM dryer reveals that not all of the PCM filled inside undergoes a phase change. This is because the PCM temperature setting is based on the lower section. When the refrigerant cools the PCM, the refrigerant enters the lower compartment of the evaporator and exits from the upper section, where the PCM temperature is higher than that of the lower part. Furthermore, the dew point required to remove moisture from the compressed air must not fall too low. As a result, a phase change occurs in the PCM in the lower section while it does not appear in the upper section. Thus, the selection criteria of the PCM to be applied to the actual PCM dryer should be based on the experimental results conducted in consideration of the operating conditions rather than the comparison of cold storage performance during complete freezing.
This study revealed that the difference in cold storage performance under the operating conditions of the actual PCM dryer was not substantial. Thus, PCM selection was based on economic feasibility. PCM B was ultimately applied to the product because the cost of PCM A used in the experiments here is 4–5 times that of PCM B.
4. Conclusions
In this study, the physical and thermal properties of two types of PCMs were investigated experimentally to select a suitable cold storage medium for a direct refrigeration PCM-type refrigerant air dryer, and based on the scope of this study, the following conclusions were drawn.
- (1) Upon the PCM being frozen, the shape is broader towards the lower section of the frozen layer; upon melting, the upper part of the frozen layer is thick and gets drastically thinner towards the lower section.
- (2) Below the freezing point, PCM A solidified homogeneously while PCM B solidified inhomogeneously, implying that PCM A is more beneficial for utilizing latent heat.
- (3) Both PCMs show a 14% change in volume during freezing and melting, which must be considered in the design process.
- (4) The dissolved gas in both PCMs occupies approximately 2% of the bulk, and most of the dissolved gas is removed when heated prior to refilling.
- (5) Under complete freezing, the cold storage performance of PCM A is higher than that of PCM B.
- (6) Only a portion of the PCM froze under PCM temperature set conditions (operating conditions of an actual PCM dryer), such that the cold storage performance difference between the two PCMs was small. Therefore, low-cost PCM B is more suitable for applications in product development.
- (7) This study ultimately selected PCM B for product development after factoring in the operating conditions and economic feasibility of the actual PCM dryer. However, applications of PCM A may be considered for the development of products such as air conditioning units that can be set to a wide range of 8 ℃ for the start of the freezing process and 0 ℃ for the melting process, because the PCM can completely freeze and melt under such PCM temperature setting.
Author Contributions
Conceptualization, M. H. Kim and B. Lee; methodology, M. H. Kim and S. K. Park; Software, S. K. Park; Formal Analysis, M. H. Kim and B. L. Kil; Investigation, B. Lee; Resources, B. Lee; Data curation H. S. Jang; Writing-Original Draft Preparation, S. K. Park and H. S. Jang; Writing-Review & Editing, M. H. Kim and B. Lee; Visualization, B. L. Kil; Project Administration, M. H. Kim; Funding Acquisition, B. Lee.
References
- SPX FLOW Technology Korea, http://spxflowkorea.com, /, accessed December 14, 2019
-
M. J. Kim, J. S. Yu, and G. H. Chea, “Fundamental study on development of latent heat storage material for waste heat recovery of biomass gasification,” Journal of the Korean Society of Marine Engineering, vol. 38, no. 5, pp. 533-540, 2014.
[https://doi.org/10.5916/jkosme.2014.38.5.533]

- M. J. Kim, “Laminar convective heat transfer from a horizontal flat plate of phase change material slurry flow,” Journal of the Korean Society of Marine Engineering, vol. 29, no. 7, pp. 779-784, 2005.
- A. D. Ukey, M. I. Khan, P. U. Bokade, and V. P. Katekar, "A reassess on phase change material application in refrigeration and air conditioning," International Journal of Mechanical Engineering Research, vol. 7, no. 2, pp. 119-128, 2017.
- F. Alzuwaid, The Novel Use of Phase Change Materials in Refrigerated Display Cabinets for Energy Conservation, Ph.D. Dissertation, Mechanical, Aerospace and Civil Engineering, Brunel University, UK, 2017.