The corrosion measurement of reinforced concrete specimens using Pt/Ti electrode
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study focused on developing a measurement method for electrochemical corrosion of reinforced concrete by using corrosion-monitoring sensors. Monitoring the corrosion of reinforced concrete structures is difficult, and therefore, this study is aimed to evaluate the electrochemical behavior of a Pt/Ti electrode for corrosion monitoring. Short-term laboratory experiments were performed to determine the corrosion rate of concrete mortar specimens under diverse corrosion conditions by using the corrosion-monitoring sensors quantitatively. Stable potentials were measured, and the corrosion rates were found to increase depending on the corrosiveness; moreover, the concrete resistivity decreased with the diffusion rate of chloride ions. Therefore, the corrosion-monitoring sensors accurately characterized the electrochemical corrosion behavior and reacted well with the severe corrosive environment.
Keywords:
Reinforced concrete, Platinized titanium, Potential, Current, Linear polarization resistance1. Introduction
Corrosion of reinforcing steel in concrete structures is an expensive corrosion problem. Rebar in concrete structures exposed to seawater is susceptible to corrosion, which can influence the life span of concrete structures in marine environments. Typically, reinforcing steel in concrete would remain indefinitely under usual atmospheric exposure as long as the concrete cover protects corrosive factors such as moisture, oxygen, and chloride from the embedded reinforcement. However, concrete structures exposed to seawater deteriorate over time due to the large amount of chloride ions in seawater. Salt damage is a critical cause for concrete bridges and harbor structures [1]. Recently, the performance of reinforced concrete structures has worsened due to severe environmental pollution and poor construction [2]. The maintenance costs for industrial infrastructure continues to increase year over year [3]. Rebar corrosion of concrete is a major parameter related to the durability of reinforced concrete structures. Generally, this type of corrosion is difficult to visualize in its early stages because the corrosion of the rebar initiates inside the concrete and propagates stealthily [4]. To establish the most economical repair methods, the corrosion of the inner reinforcement must be accurately and quantitatively detected at an early stage. Significant efforts have been devoted to corrosion measurement of reinforced concrete in an attempt to develop a reliable technology, but no satisfactory methodology has been reported to date [5]. Corrosion of reinforced concrete structures is a sensitive phenomenon that depends on many factors including the concrete cover and grade, temperature, humidity, carbonation, and chloride contamination. This multiplicity of factors significantly influences the data obtained based on either in-situ observations or experimental investigations [6]. Comparing the several hundred studies regarding the corrosion of reinforced concrete, corrosion data is often obtained from a variety of experimental setups and parameters. Almost all of the reported data were obtained from laboratory studies, whereas only several studies have reported long-term field tests in-situ. This large difference in the literature is related to the different experimental procedures, measurement methods, and variety of parameters that affect the corrosion of steel in concrete. Stefanoni et al. concluded that the main parameter controlling the corrosion rate of steel in concrete is exposure conditions, and an inverse relation between concrete resistivity and corrosion rate provided an empirical correlation depending on the degree of concrete pore saturation [7]. Corrosion measurement techniques using electrochemical methods have attracted attention as accurate and fast non-destructive technologies.
However, many difficulties including the interpretation of results, environmental effects, and problems with measurement methods for the use of appropriate electrochemical methods on reinforced concrete structure persist.
To date, two major methods for accurately measuring the corrosion of reinforced concrete have been developed: measuring from the outside of the concrete structure or using embedded sensors [8].
Corrosion sensors that feature half-cell potential mapping which measures corrosion potential of rebar at the surface of the concrete are widely used. These sensors are useful for rapid and facile surveying and evaluation of rebar corrosion with simple measurement equipment in the field. For embedded sensors, measurements of current density, corrosion rate, concrete resistivity, and chloride content can be achieved [9].
Herein, electrochemical measurements of embedded corrosion-monitoring sensors, including corrosion potential, corrosion current density, concrete resistivity, and potentiodynamic polarization tests were performed to investigate the corrosion behavior of reinforced concrete over time both qualitatively and quantitatively.
2. Experimental Method
2.1. Corrosion-monitoring sensors
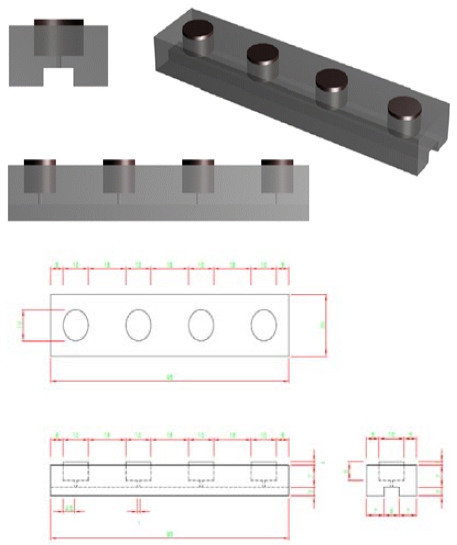
The corrosion-monitoring sensors were fabricated for measuring corrosion in a cement mortar specimen. The sensor consisted of a platinized titanium (Pt/Ti) electrode, stainless steel electrode, and 2 steel electrodes in cement mortar.
Figure 1 shows a schematic drawing of the corrosion-monitoring sensor with dimensions of 95 × 20 × 10 mm. The working, counter, and reference electrodes were installed in a Teflon fitting mold. The electrodes were 10 mm in diameter with a thickness of 6 mm. Only the top surface of all electrodes was exposed with a 10 mm diameter and the other surfaces were inserted into the Teflon mold. All lead wires for measuring the corrosion behavior were connected to the electrode through the lower small hole with a diameter of 1 mm and sealed with epoxy. A working electrode composed of mild steel, stainless steel 316L auxiliary electrode next to the working electrode for current distribution between the two electrodes, and Pt/Ti as a reference electrode were used. These materials were chosen due to their reliability because of the exposure to the highly alkaline concrete conditions for long periods. The distance between the electrodes was 15 mm, and the thickness of the edge of the electrode at both ends was 5 mm.
2.2. Cement mortar specimens
Cement mortar specimens are typically formed using a combination of cement, sand, and water at a 1 : 2 : 0.5 wt. ratio. The material consisted of a mortar with a water/cement ratio of 0.5. A cylindrical specimen 80 mm in diameter and 160 mm in length was fabricated. The cement mortar specimens were exposed to corrosion conditions, i.e. atmospheric, tap water, 3% saltwater, and 15% saltwater conditions after curing for 30 days. The atmospheric condition specimens were exposed to laboratory conditions and the others were continuously immersed in test solutions. A total of 12 specimens, 3 specimens for each experimental condition, were fabricated.
2.3. Test methods
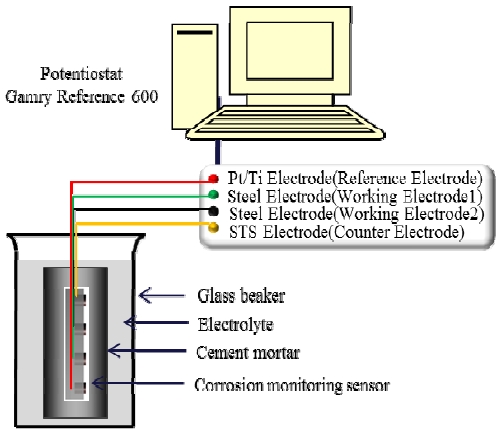
The electrochemical measurements were performed using a sensor with a half-cell potential as a function of time to examine stability under highly alkaline environments. Corrosion rates were measured by potentiodynamic polarization to quantitatively analyze the corrosion behavior. To measure the electrochemical behavior, corrosion potential measurements of the Pt/Ti reference electrode in the cylindrical mortar specimen exposed to each corrosion condition was measured using an Ag/AgCl reference electrode and high impedance fluke voltmeter [10]. Corrosion current density was calculated by algorism of a Gamry Instruments Reference 600 potentiostat [11]. Linear polarization tests were performed to calculate the corrosion rate and corrosion current density using corrosion-monitoring sensors imbedded in cement mortar specimens exposed to a variety of corrosive environments. A method used to determine corrosion rate involves measuring the weight loss, as it is very simple, but takes a long time to obtained results. Potentiodynamic polarization tests were performed to examine the anodic polarization behavior of the working electrode in the sensor. The scan range was from -100 mV (below the stable potential) to +1,500 mV at a scan rate of 5 mV /s. Concrete resistance was measured using a Nilsson soil resistivity meter via the 4-pin method [12].
Figure 2 shows a schematic of the experimental setup with a cylindrical cement mortar specimen in the test solution. A corrosion-monitoring sensor embedded in the cylindrical cement mortar was connected to the potentiostat. The experiments were performed under atmospheric, tap water, 3% saltwater, and 15% saltwater conditions at 25 °C to examine the chloride effect.
3. Experimental Results
Figure 3 shows the half-cell potential measurements between the embedded Pt/Ti reference electrode probe and Ag/AgCl reference electrode. These results showed potential variations of the Pt/Ti reference electrode in the cylindrical mortar specimen exposed to three corrosive conditions, i.e. tap water, 3% saltwater, and 15% saltwater with a silver/silver chloride reference electrode. The half-cell potential measurements were performed over 10 days. Variations of the potential of the embedded Pt/Ti reference electrode ranged from -100 to -130 mV under all environments. Thus, this setup was suitable for use as a reference electrode since the deviation of the half-cell potential was small. Thus, it was consider being usable as a reference electrode embedded in mortar specimen.
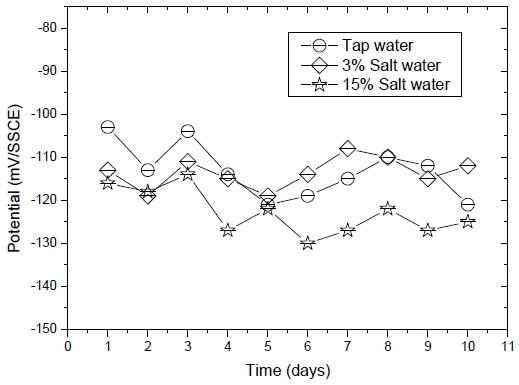
Potential measurements of the Pt/Ti reference electrode in the cylindrical mortar specimen exposed to various corrosion conditions
Figure 4 shows a comparison of the obtained corrosion current densities under four test conditions. The corrosion current densities measured using the sensors imbedded in the mortar specimens exposed to atmospheric and tap water conditions were negligible at approximately 4.2 × 10-4 mA/cm2 over the course of the measurement. However, the current densities of the mortar specimens exposed to 3% and 15% saltwater conditions were significantly higher due to the absorbed salinity from the saltwater. The current density under the 15% saltwater conditions (the most saline environment) was significantly increased to 1.1 × 10-1 mA/cm2 after 90 days of exposure.
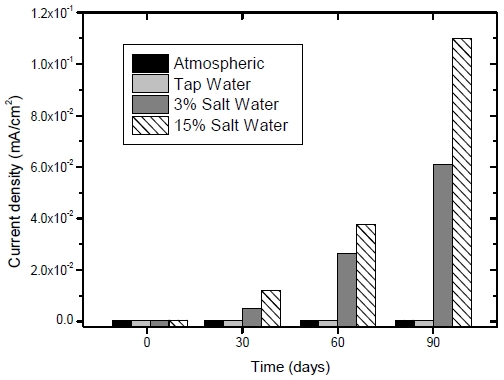
Current density measurements of the sensor in the cylindrical mortar specimen exposed to various corrosion conditions
The magnitudes of the current densities under the atmospheric and tap water conditions were the smallest, followed by the 3% saltwater condition, and the 15% saltwater condition was the largest, similar to previously reports [13]. The sensor with a Pt/Ti reference electrode provided good indication of corrosion status when applied to the reinforced concrete structure via current density results.
The concrete resistivity measurement was performed simultaneously with the corrosion rate test. Figure 5 shows the concrete resistivity of the sensor embedded in the mortar specimens under the four test conditions. Under atmospheric conditions, the concrete resistivity was increased to approximately 1.3 kohm·cm over time due to the hydration reaction of the cement. Under the other conditions, i.e. tap water, 3% saltwater, and 15% saltwater conditions, the concrete resistivities were noticeably lower because of the conductive environment. In particular, the 15% saltwater condition exhibited the lowest resistivity of all conditions due to the diffusion rate of chloride ions induced by the large concentration difference.
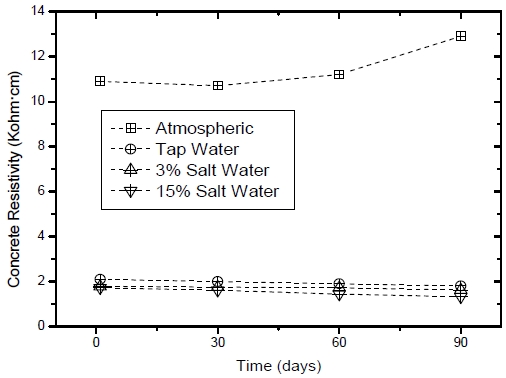
Concrete resistivity results of the sensor embedded in the cylindrical mortar specimens exposed to various corrosion conditions
Figure 6 shows the potentiodynamic polarization tests results. Under atmospheric and tap water conditions, positive corrosion potentials and reduced current densities were observed in the polarization diagram. In addition, the corrosion current density was lower than those of the saltwater conditions, and the pitting potential where the current density significantly increases at a noble potential was not observed. Thus, it was assumed that a dense passive layer on the steel surface of the sensor was formed due to the high alkalinity of the cement mortar and absence of chloride ions.
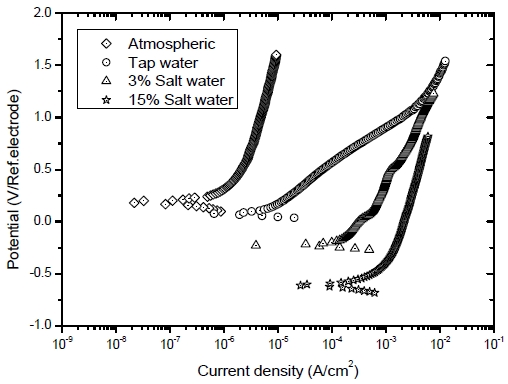
Potentiodynamic polarization results of the sensor embedded in the cylindrical mortar specimens exposed to various corrosion conditions
The sensors under the 3% and 15% saltwater conditions exhibited corrosion behavior with higher current densities approximately 104 times higher than that observed under atmospheric conditions. The corrosion current density of the sensor exposed to 15% saltwater conditions was enormously high. It was assumed that the chloride ions in saltwater penetrated into the specimen, breaking the passive film on the metal surface of the sensor.
From the electrochemical behavior results, the corrosion-monitoring sensors embedded in the cement mortar specimen could effectively monitor the corrosion behavior of reinforced concrete over time both qualitatively and quantitatively.
4. Conclusion
From the experimental results regarding the electrochemical behavior of the corrosion-monitoring sensors, the corrosion-monitoring sensors embedded in the cement mortar specimens effectively monitored the corrosion behavior of the reinforced concrete over time both qualitatively and quantitatively. The major conclusions of this study are as follows.
- (1) The corrosion potential of the Pt/Ti reference electrode embedded in the mortar specimen was measured using a silver/silver chloride reference electrode over 10 days. The corrosion potential ranged from -100 to -130 mV in all environments tested.
- (2) The corrosion rate as measured by the linear polarization resistance test increased in order of environmental severity with increasing chloride content in the mortar specimen.
- (3) Concrete resistivity decreased with increasing diffusion rate of chloride ions induced by concentration differences.
Acknowledgments
This work was supported by the Korea Maritime & Ocean University Research Fund.
Author Contributions
Conceptualization, J. A. Jeong and M. Kim; Methodology, J. A. Jeong; Software, J. A. Jeong; Validation, J. A. Jeong and M. Kim; Formal Analysis, J. A. Jeong; Investigation, J. A. Jeong; Resources, J. A. Jeong; Data Curation, J. A. Jeong; Writing-Original Draft Preparation, J. A. Jeong; Writing-Review & Editing, M. Kim; Visualization, J. A. Jeong; Supervision, M. Kim; Project Administration, M. Kim; Funding Acquisition, M. Kim.
References
- J. A. Jeong, M. S. Kim, S. D. Yang, C. H. Hong, N. K Lee, and D. H. Lee, “Cathodic protection using insoluble anodes by delivering protection currents the inner surfaces of carbon steel seawater pipes”, Journal of the Korean Society of Marine Engineering, 42(4), p280-286, (2018), (in Korean).
- S. Ahmad, “Reinforcement corrosion in concrete structures, its monitoring and service life prediction - A review”, Cement and Concrete Composites, 24(4), p459-471, (2003).
- A. A. Sagues, and R. G. Powers, “Sprayed-Zinc sacrificial anodes for reinforced concrete in marine service”, Corrosion, 52(7), p509, (1996).
- H. W. Song, and V. Saraswathy, “Corrosion monitoring of reinforced concrete structures - A review”, International Journal of Electrochemical Science, 2(1), p1-28, (2007).
-
S. Qian, D. Cusson, and N. Chagnon, “Evaluation of reinforcement corrosion in repaired concrete bridge slabs-A case study”, Corrosion, 59(5), p457-468, (2003).
[https://doi.org/10.5006/1.3277577]

-
E. S. Cavaco, A. Bastos, and F. Santos, “Effects of corrosion on the behaviour of precast concrete floor systems”, Construction and Building Materials, 145, p411-418, (2017).
[https://doi.org/10.1016/j.conbuildmat.2017.04.044]

-
M. Stefanoni, U. Angst, and B. Elsener, “Corrosion rate of carbon steel in carbonated concrete-A critical review”, Cement and Concrete Research, 103, p35-48, (2018).
[https://doi.org/10.1016/j.cemconres.2017.10.007]

- D. Bjegovic, J. J. Meyer, D. Mikulic, and D. Sekulic, “Corrosion measurement in concrete utilizing different sensor technologies”, NACE, Corrosion Conference, Paper No. 03435, (2003).
- T. S. Nguyen, S. Lorente, and M Carcasses, “Effect of environment temperature on the chloride diffusion though CEM-I and CEM-V mortars: an experimental study”, Construction and Building Materials, 23(2), p795-803, (2009).
-
B. Elsener, C. Andrade, J. Gulikers, R. Polder, and M. Raupach, “Half-cell potential measurements-potential mapping on reinforced concrete structures”, Materials and Structures, 36(7), p461-471, (2003).
[https://doi.org/10.1007/bf02481526]

- J. E. Alfonso, J. J. Olaya, M. J. Pinzon, and J. F. Marco, “Potentiodynamic polarization studies and surface chemical composition of bismuth titanate films produced through radiofrequency magnetron sputtering”, Materials, 6, p4441-4449, (2013).
-
K. Hornbostel, C. K. Larsen, and M. R. Geiker, “Relationship between concrete resistivity and corrosion rate—A literature review”, Cement and Concrete Composites, 39, p60-72, (2013).
[https://doi.org/10.1016/j.cemconcomp.2013.03.019]

-
S. Karthick, S. Muralidharan, H. S. Lee, S. J. Kwon, and V. Saraswathy, “Reliability and long-term evaluation of GO-MnO2 nano material as a newer corrosion monitoring sensor for reinforced concrete structures”, Cement and Concrete Composites, 100, p74-84, (2019).
[https://doi.org/10.1016/j.cemconcomp.2019.03.012]