An improved battery-impedance measurement module for electric propulsion ships and a study of battery characteristics
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The International Maritime Organization has recently strengthened the regulation of air pollutants, and battery-powered eco-friendly electric propulsion vessels have drawn attention. An electric ship's main power unit is an energy storage system (i.e., batteries). For propulsion, attempts have been made to use lithium-ion batteries with high energy densities as secondary power sources. Unfortunately, these batteries present a higher risk of explosion than primary lead-based batteries. In recent years, electrochemical impedance spectroscopy, which measures the impedance of a battery and predicts its lifetime, has been widely used to mitigate this risk. Extant EIS equipment is large and expensive, however. Thus, it is inefficient and uses push-pull circuitry, resulting in distorted output voltages. In this paper, we fabricate an EIS impedance measurement module that minimizes this distortion and measure the impedance parameters of a battery when charging and discharging. Then, we analyze the battery’s aging and lifetime.
Keywords:
Lithium battery, Electrochemical impedance spectroscopy, Distortion1. Introduction
The International Maritime Organization has pushed emission reductions by introducing regulations for greenhouse gases and other pollutants. Research and commercialization of eco-friendly vessels, such as electric propulsion ships and fuel-cell ships, is under way. These vessels use electronic storage system (ESS) devices containing batteries. In recent years, several attempts have been made to apply an ESS using high energy density lithium batteries [1][2].
However, because energy storage technologies for batteries are unsuitable for long-time, long-distance ships, researchers are actively pursuing hybrid systems (e.g., internal combustion engines, fuel cells, and ESSs). However, lithium-ion batteries and lithium-polymer batteries have a higher risk of explosion and fire than other primary and secondary batteries. Thus, battery management must be thoroughly performed [1][2].
It is very important to check the state of charge of a lithium-ion battery. Owing to electrochemical problems inside the battery, aging can proceed suddenly, leading to an explosion. Additionally, when a battery is continuously charged and discharged, its life and capacity are quickly reduced. If one battery in the ESS drops capacity, the utility of the entire unit can weaken. By frequently analyzing the battery characteristics of each cell, it becomes possible to ensure the safety of the entire system by enabling the quick replacement of aging batteries [2][3].
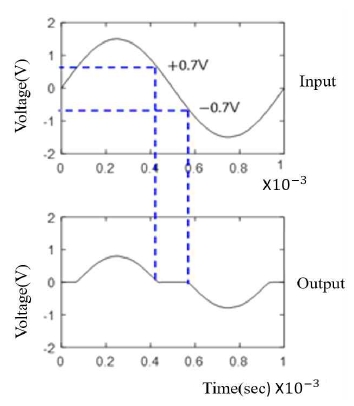
Recently, equipment for analyzing aging and life characteristics of batteries has been studied. This equipment estimates R and X values using electrochemical impedance spectroscopy (EIS). However, this equipment is not easily applied, because it is expensive and bulky. EIS measures the internal impedance of a battery by injecting a small alternating current (AC) voltage into it. The circuit for generating an AC voltage waveform uses a push-pull method. However, as shown in Figure 1, crossover distortion occurs in the AC voltage waveform, caused by the method. This is unsuitable for impedance measurement. Therefore, a filter is needed.
In this paper, we develop a simple circuit that can minimize the waveform distortion of the AC voltage injected into the battery. We also analyze aging characteristics and battery lifetime by fabricating a module for measuring internal impedance.
2. Battery theory
2.1 Internal parameters
The lithium battery changes voltage according to the SOC. The lowest safe battery level is SOC 0%, and the maximum is 100%. The stored capacity of a battery is defined as the total amount of discharge occurring when a constant current is discharged from a fully-charged state to a fully-discharged state. The current battery (SOC) can be obtained by using the Coulomb counting method. The formula is as follows.
| (1) |
SOCbat0 is a value obtained by integrating the initial value of the battery and the measured current. The latter part of the equation contains the integral of the measured discharge current divided by the discharge capacity. This method is commonly used for measuring battery SOC at a very high accuracy, provided there are no errors [4][5].
For a battery with a capacity of 1 Ah, the reference 1C charging rate (1 hr) is 1 A, and the amount of current is the capacity of the cell. This unit is applied to both charge and discharge. The manufacturer displays the maximum charge current and maximum discharge current as A. Otherwise, they use a C rating [5].
A new battery is defined as SOH 100%, and a battery that has reached its end of life using a battery is defined as SOH 0%. Predicting the current state of the battery and estimating its available time can improve reliability and prevent accidents.
However, accurately estimating SOH is difficult, because batteries store and use energy via complex chemical reactions. The SOH estimation of a battery leverages a method of estimating the capacity and internal impedance [2][5].
2.2 EIS
The electrical method deals with current and voltage. The chemical method deals with the change of constituent materials.
The electrochemical method, thus, can effectively explain simple phenomena via the relation of voltage, current, and chemistry.
Unfortunately, the electrochemical method cannot obtain enough information about a simple voltage–current relationship when it is applied to a battery that uses a complicated reaction. The complementary method needed is EIS. This method measures the voltage–current in response to frequency and analyzes the impedance [4][6][7].
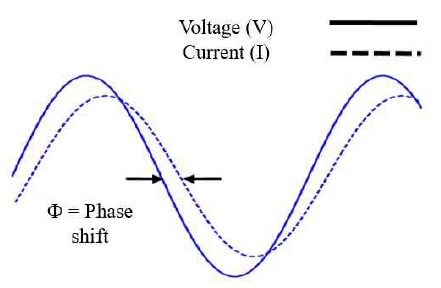
When a small AC voltage signal (V) is injected into a battery, an AC current (I) is generated according to the battery’s internal impedance, which causes a phase difference in the AC current. This phase difference is called a “phase shift.” [8]
Impedance is represented by a complex number (R + jX). R represents the resistance component, and X represents the reactance component. Impedance, Z, is expressed as follows:
| (2) |
The relationship between the sinusoidal voltage and the response current is shown in Figure 2[6].
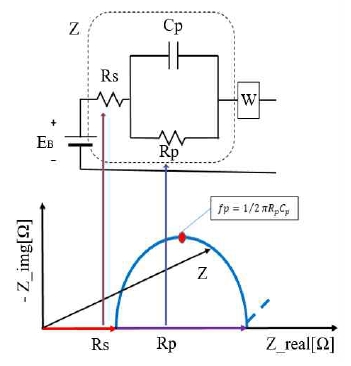
Figure 3 shows the internal impedance circuit of the battery and the EIS graph. Rs is the internal resistance of the battery. At high frequencies, Cp is shorted, and only Rs measured. As the aging process progresses, the value of Rs rises. This is used as a criterion for determining battery life. Rp is the ionization loss resistance caused by the current. Cp is a capacitor function created by the electric double layer between the electrode and the electrolyte. Because the voltage accumulates after a certain frequency, current can flow in Rp, and a value can be measured. The Rp value, according to the frequency, can be continually measured to observe the reaction rate according to the ionization loss. Rp and Cp are thus used to determine the SOC of the battery [4][8].
3. EIS module configuration
3.1 Hardware
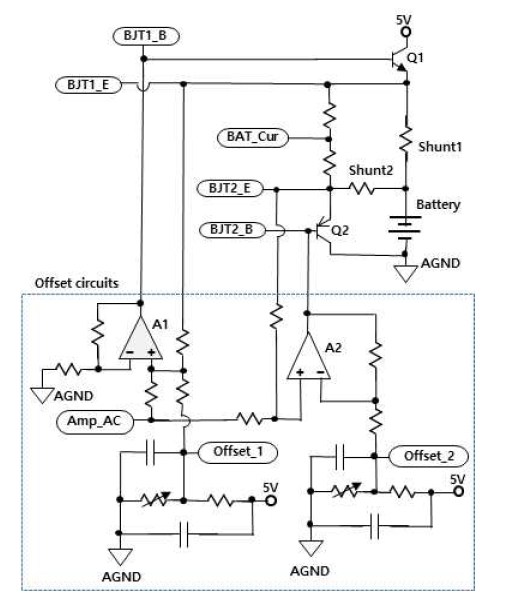
Figure 4 shows the proposed AC-voltage injection circuit and offset-adjustment circuit for EIS. The AC voltage injection circuit injects AC voltage into the battery and measures the current. The shape of the push-pull circuit is modified. A PNP transistor uses three separately doped regions with two junctions: one N region sandwiched between two P regions. An NPN has one P region sandwiched between two N regions. A bipolar junction transmitter circuit is designed connecting a shunt resistor to one PNP and one NPN to measure the current.
The existing push-pull circuit operates by injecting a single input signal that creates crossover distortion in the output AC voltage. A circuit is needed to offset this distortion. The circuit in the dotted rectangle of Fig. 3 is the proposed offset circuit. The frequency injected is input as micro AC signal, Amp_AC, superimposed on the BJT1_E terminal, which is the turn-on reference of Q1. Offset1 is added, so that BJT1_B is higher than BJT1_E from the turn-on voltage. Additionally, if Amp_AC is superimposed on the BJT2_E terminal, the turn-on reference of Q2 and BJT2_B is injected to the push-pull circuit by subtracting Offset2 from BJT2_E from the turn-on voltage. An AC voltage waveform without distortion is thus created.
The separated waveform is then input into the push-pull circuit to produce a distortion-free AC voltage waveform. Conventional crossover distortion phenomenon is proposed using a diode. However, for this paper, a sinusoidal AC voltage that does not become distorted waveform is output through the offset circuit.
3.2 Software
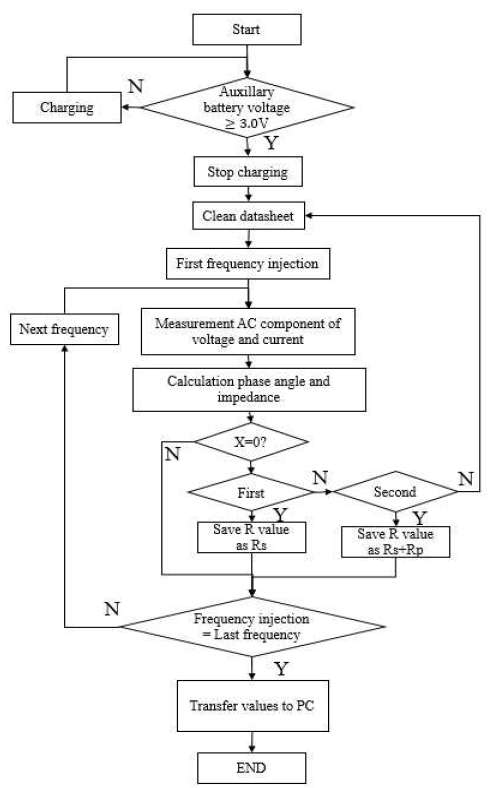
Figure 5 presents an algorithm that measures the impedance of the battery running on the microcontroller unit (MCU). First, we check the battery voltage. If above 3.0 V, we stop charging and inject the first frequency of current into the battery from the MCU. When the frequency is injected, the voltage and current are measured after a slight delay.
The resistance component, R, and the reactance component, X, are obtained by measuring a change value of a current, a change value of a voltage, and a phase angle between the voltage and current. This angle is obtained by multiplying the applied frequency by the time difference between the points where the voltage and the current become zero, respectively.
If the initial X value of the reactance value is zero, it is calculated as Rs. If the resultant X value is 0, it is calculated as Rs + Rp. We repeat the above steps while decreasing the frequency one-by-one to continuously measure impedance. It is possible to analyze the battery life and state by expressing the value measured sequentially by the user in the frequency band specified by the impedance graph.
4. Experiment
4.1 Device configuration of experiment
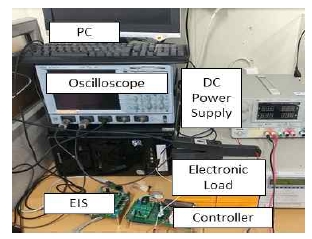
Figure 6 shows the experimental device, where the lithium polymer battery is charged by a direct-current power supply. An electronic load is used for discharging at a constant current. The battery model and specifications are shown in Figure 7 and Table 1, respectively.
To measure Rs, the battery was charged by the MCU when the voltage reached 3.0 V and discharged when the voltage reached 4.2 V. After 100 charge/discharge cycles, the battery was measured using the EIS module. The charge amount of the battery was adjusted according to the SOC by the controller to examine the change of the value with respect to the charging state.
4.2 Experimental application of system
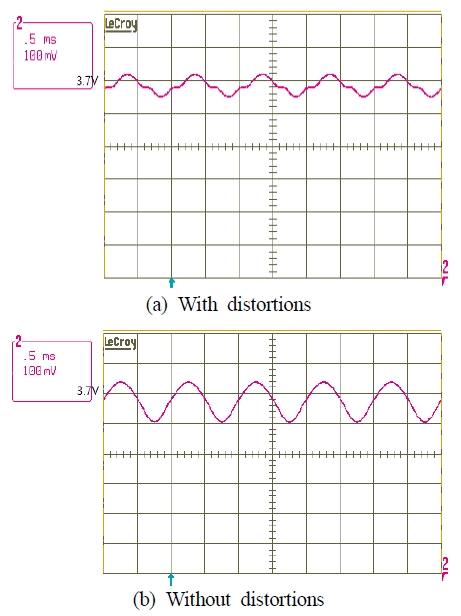
The circuits in Figure 4 were used to measure the AC voltage signal injected into the battery. Thus, the sinusoidal AC voltage signal without distortion is equal to that shown in Figure 8 (b). Distortion occurred in the range of + 0.04 V to -0.04 V, and the peak-to-peak deviation from the distorted waveform and the improved sinusoidal waveform was 0.08 V.
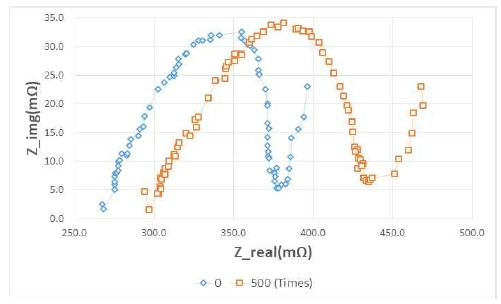
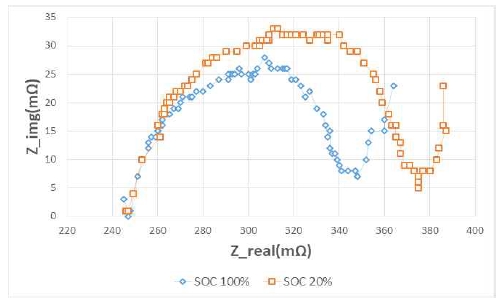
Figure 9 and Figure 10 show the changes in impedance when the frequency is changed from 5 kHz to 50 mHz to confirm the impedance characteristic theory mentioned in Section 2. [8] The left graph in Figure 9 displays the new battery, and the right side displays the battery after 500 rapid charge/discharge cycles.
The Rs value is measured largely on the battery used for longer than the new battery. From this result, it can be seen that the value of R1 gradually increases as the battery is used.
Figure 10 shows that Rp was measured to be larger at SOC 20% than at 100%. These results show that the value of Rp increased in accordance with the decrease of SOC. Therefore, as the value of Rp increases, the capacity decreases.
5. Conclusion
In this paper, we developed a measurement circuit that easily applies EIS without causing voltage distortion. We measured the internal equivalent impedance of the battery and analyzed the aging and the life of the battery. The impedance of the lithium polymer battery was measured as applied to the proposed module. The following conclusions were obtained.
- (1) Resulting from the experiment using the proposed circuit, a good sinusoidal AC voltage signal without distortion of the AC voltage signal was obtained by the manufactured EIS equipment.
- (2) As the number of charge/discharge cycles increased, the Rs value increased, and the aging process accelerated. As the amount of SOC decreased, the value of Rp increased, and the battery capacity decreased.
- (3) The proposed EIS measurement equipment will contribute to the safety of ships under weigh, because it is easy to analyze battery status and information via the impedance graph.
In the future, we expect to apply a system to identify bad batteries.
Acknowledgments
Following are results of a study on the “Leaders in INdustry-university Cooperation +” Project, supported by the Ministry of Education and National Research Foundation of Korea, and this research was an improvement of Song Tae-hyun's master's thesis (“The study on the charge cycle for optimization of battery pulse charging, Graduate school of Korea Maritime and Ocean University, Korea, 2019”).
Author Contributions
The following statements should be used “Conceptualization, T. H. Song and S. G. Lee; Methodology, T. H. Song and J. L. Choi; Software, J. L. Choi and H. S. Yang; Validation, T. H. Song and J. L. Choi; Investigation, J. L. Choi and Y. S. Kim; Resources, T. H. Song and H. S. Yang; Data Curation, T. H. Song and H. S. Yang; Writing—Original Draft Preparation, T. H. Song; Writing—Review & Editing, T. H. Song and H. S. Yang; Supervision, S. G. Lee; Project Administration, T. H. Song and H. S. Yang; Funding Acquisition, T. H. Song.
References
- H. J. Kwon, A Study of Remote Management System of Lithium Ion Battery for Ship Based on BLE, M.S. Dissertation, Department of Electrical and Electronic Engineering, Graduate School of Korea Maritime and Ocean University, Korea, (2015), (in Korean).
- H. S. Ma, Battery Management System with High-Reliability for Power Conversion System, M.S. Dissertation, Department of Electrical and Electronic Engineering, Graduate School of Korea Maritime University, Korea, (2017), (in Korean).
- J. L. Choi, Study on Electrochemical Impedance Spectroscopy Equipment for Checking State of Battery, M.S. Dissertation, Department of Electrical and Electronic Engineering, Graduate School of Korea Maritime and Ocean University, Korea, (2018), (in Korean).
- S. I. Kong, Battery Modeling and SOC Estimation Using Extended Kalman Filter, M.S. Dissertation, Department of Electrical Engineering, Chungnam National University, Korea, (2013), (in Korean).
-
T. H. Song, H. S. Yang, Y. S. Kim, S. G. Lee, “Investigation of the lifespan characteristics of a rapid charge/discharge battery using the inverter level discharge method”, Journal of the Korean Society of Marine Engineering, 42(7), p615-619, (2018), (in Korean).
[https://doi.org/10.5916/jkosme.2018.42.7.615]

- Y. M. Jeong, An Enhanced OCV Reset Algorithm to Improve the Coulomb Counting Method of Li-Polymer Battery for xEVs, M.S. Dissertation, Department of College of Information & Communication Engineering, Sungkyunkwan University, Korea, (2015), (in Korean).
- G. H. Lee, Development of the SOC Estimation Method of the Battery and the Battery Inspection System using Electrochemical Impedance Spectroscopy, M.S. Dissertation, Department of Electrical and Electronic Engineering, Graduate School of Soongsil University, Korea, (2010), (in Korean).
- T. H. Song, The Study on the Charge Cycle for Optimization of Battery Pulse Charging, M.S. Dissertation, Department of Electrical and Electronic Engineering, Graduate School of Korea Maritime and Ocean University, Korea, (2019), (in Korean).