Fabrication of CuCo(OH)2/graphene paper electrode and their application for anion exchange membrane water electrolysis
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In this study, we synthesized CuCo(OH)2 on graphene (Gr) paper via electrodeposition and explored its effectiveness as a hydrogen evolution reaction (HER) electrode for anion exchange membrane (AEM) electrolysis. The resulting CuCo(OH)2 exhibited a nanosheet morphology, increasing the surface area for electrochemical processes, thereby enhancing the extrinsic activity. Compared to the AEM electrolysis cell equipped with Co(OH)2/Gr, that equipped with CuCo(OH)2/Gr exhibited superior efficiency, highlighting its potential for sustainable hydrogen production.
Keywords:
Anion exchange membrane water electrolysis, Water splitting, Hydrogen energy, Electrocatalysis1. Introduction
Hydrogen energy is an eco-friendly, sustainable energy source with a high energy density and does not emit greenhouse gases; it is attracting attention in the context of accelerating global warming [1]. Among the various hydrogen production technologies, water elec-trolysis, which entails the use of electricity to split water into hydrogen and oxygen, is considered the most environmentally friendly [2]. The water electrolysis reaction involves two distinct processes: the oxygen evolution reaction (OER), an oxidation reaction, and the hydrogen evolution reaction (HER), a reduction reaction [3]. Each of these reactions requires considerable overpotential. Therefore, efforts are necessary to minimize overpotential and achieve high-efficiency water electrolysis. In particular, because the HER is a key reaction that generates hydrogen during water electrolysis, reduction of its overpotential is important. This necessitates the development of highly active electrocatalysts for HER.
Cobalt, known for its activity as indicated by its HER volcano plot, is a prominent material used in the catalysts for HER. Copper also exhibits catalytic activity and excellent conductivity. Consequently, substitution with Cu can enhance the conductivity and modify the electrical structure of Co, thereby promoting the HER.
Recently, there has been a growing interest in anion exchange membrane (AEM) water electrolysis (AEMWE) technology, which uti-lizes cost-effective non-precious metals (non-PGMs) and can afford high efficiencies at high current densities [4]. This next-generation water electrolysis technology combines the advantages of alkaline water electrolysis (AWE) and proton-exchange membrane (PEM) water electrolysis [5]. However, the commercialization of AEMWE has been hindered by its low efficiency, primarily because of the high overpotential of both the OER and HER due to insufficient catalytic activity [6].
In this study, we developed a highly active non-PGM-based HER electrocatalyst (CuCo(OH)2) for AEM electrolysis. CuCo(OH)2 was synthesized directly on graphene (Gr) paper via electrodeposition. The synthesized CuCo(OH)2 exhibits fine nanosheet shape and yields a large surface area for electrochemical reactions. The incorporation of Cu into Co(OH)2 modified the electronic structure of Co, resulting in enhanced HER activity. In addition, the AEM electrolysis cell equipped with CuCo(OH)2/Gr exhibited a higher efficiency than that equipped with Co(OH)2/Gr.
2. Experimental
2.1 Preparation of Co(OH)2/Gr and CuCo(OH)2/Gr
CuCo(OH)2/Gr was prepared via electrodeposition performed using a three-electrode system [7]. A saturated calomel electrode (SCE) and titanium felt were used as the reference and counter electrodes, respectively. The Gr paper, used as the working electrode, was pre-pared by grinding Gr powder by using a mortar. An aqueous solution of 10 mM Cu(NO3)2 and 50 mM Co(NO3)2 was used as the elec-trolyte for electrodeposition. A potential of -1 VSCE was applied to the working electrode for 5 min. After electrodeposition, the samples were washed with deionized (DI) water and dried at room temperature. Co(OH)2/Gr was prepared by applying a potential of -1 VSCE to the working electrode for 5 min in a solution containing 50 mM Co(NO3)2.
2.2 Characterization of Physical Properties
The surface morphology was examined via field-emission scanning electron microscopy (FE-SEM; CZ/MIRAI LMH, TESCAN). The crystal structures of the samples were analyzed using X-ray diffraction spectroscopy (XRD, Ultimalv, Rigaku) in the scattering angle (2θ) range of 5°–80°.
2.3 Electrochemical Characterization
Electrochemical measurements were performed using a three-electrode system with a potentiostat (ZIVE MP1, WonATech). The pre-pared samples were used as working electrodes. A graphite rod and Hg/HgO (in 1 M KOH) were used as the counter and reference electrodes, respectively. A solution (1 M KOH) was used as the electrolyte and purged with N2 gas for 30 min to eliminate the effect of the dissolved oxygen gas [8]. The performance of the prepared electrode for HER was assessed using linear sweep voltammetry (LSV) at a scan rate of 5 mV/s with iR correction. All potentials were converted to the reversible hydrogen electrode (RHE) potential by using the Nernst equation [9]. Electrochemical impedance spectroscopy (EIS) was conducted at -0.250 VRHE over frequencies ranging from 100 kHz to 1 Hz, with a signal amplitude of 10 mV.
2.4 AEM Electrolyzer Testing
AEM electrolyzer comprised a flow channel (Ti and graphite at anode and cathode), a gold-plated current collector, an AEM (Sustain-ion®X37-50 Grade T, Dioxide Materials), and an end plate. The prepared CuCo(OH)2/Gr and Co(OH)2/Gr composites were used as cathodes. The anodes were prepared using a spraying method. An ink solution for spraying was prepared by mixing NiFe layered dou-ble hydroxide (NiFe-LDH) powder (OER catalysts), DI water, isopropyl alcohol, and an ionomer (Fumion®FAA-3-solut-10). The NiFe-LDH was synthesized based on previous studies [10]. The loading mass of NiFe-LDH was 5 ± 10% mg/cm2. The active area of AEM electrolyzer was 0.785 cm2, and the operating temperature was approximately 55 ℃. KOH solution (1 M) was supplied to the AEM electrolyzer at a flow rate of 50 mL/min. The cell voltage was measured as a function of the current density by using a DC power supply (MK-W102, MKPOWER) to obtain the polarization curves of the AEM electrolyzer. The durability of the AEMWE was tested at a current density of 0.5 A/cm2 for 60 h. The cell efficiency was determined using the following equation:
| (1) |
where ΔHH2,LHV is the lower heating value reaction enthalpy for water electrolysis (241.8 kJ mol-1), nH2,measured is the hydrogen produc-tion (mol s-1) measured using the DC power supply, I is the applied current (A), and V is the applied voltage (V) [11].
3. Results and Discussion
3.1 Catalyst Synthesis and Structure Characterization of Samples
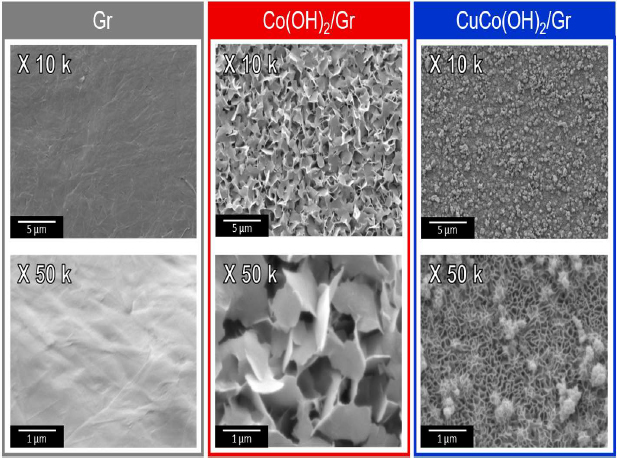
CuCo(OH)2/Gr were analyzed using scanning electron microscopy (SEM). The surface morphology of Co(OH)2/Gr showed rough and large nanosheets. Conversely, CuCo(OH)2/Gr showed a fine nanosheet, which provided a larger surface area to promote electro-chemical reactions (Figure 1) [12].
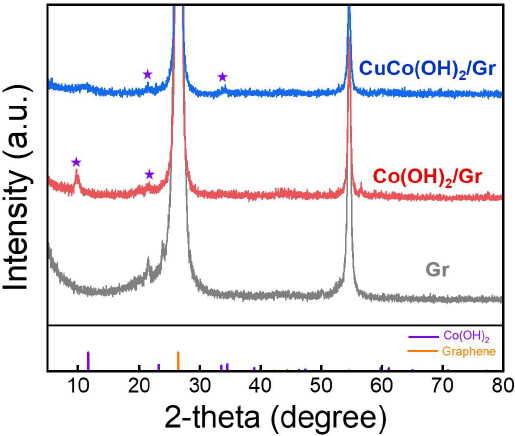
XRD patterns were obtained to confirm the crystal structures of Gr, Co(OH)2/Gr, and CuCo(OH)2/Gr (Figure 2). For Co(OH)2/Gr and CuCo(OH)2/Gr, distinct peaks corresponding to the (003) and (006) planes of Co(OH)2 are observed at 11° and 22°, respectively [13]. Interestingly, the crystallinity of CuCo(OH)2/Gr was lower than that of Co(OH)2/Gr because it entailed the simultaneous nucleation of different metal ions to form hydroxides.
3.2 Electrochemical Analysis of Gr, Co(OH)2/Gr, and CuCo(OH)2/Gr
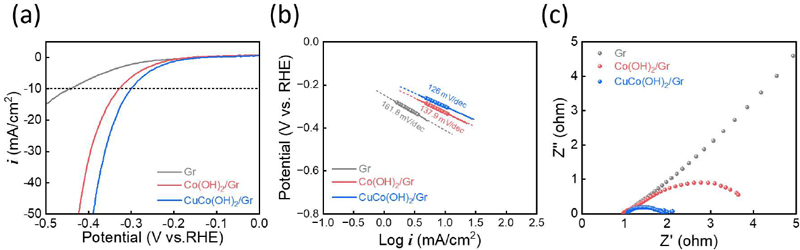
The electrode performances for HER were evaluated using a three-electrode system in 1 M KOH purged with N2 gas. The overpoten-tial at the current density of -10 mA/cm2 of Gr, Co(OH)2/Gr, and CuCo(OH)2/Gr showed 440 mV, 328 mV, and 300 mV, respectively. These results indicate that CuCo(OH)2/Gr is the best HER electrode (Figure 3a). The introduction of Cu into Co(OH)2 modified the electronic structure of Co, leading to an improvement in the intrinsic HER activity. In addition, the fine nanosheet improved the extrinsic HER activity. As a result of this synergistic effect, CuCo(OH)₂/Gr exhibited the best HER performance. The Tafel slope of CuCo(OH)2/Gr is the lowest at 126 mV/dec, indicating that CuCo(OH)2/Gr exhibits the fastest HER kinetics (Figure 3b). A Nyquist plot was used to investigate the polarization resistance of the HER. The radius of the semicircle in the Nyquist plot indicates the polariza-tion resistance [14]. This was the smallest for CuCo(OH)2/Gr; therefore, CuCo(OH)2/Gr exhibited the best HER performance (Figure 3c).
3.3 Testing of AEM Electrolyzer
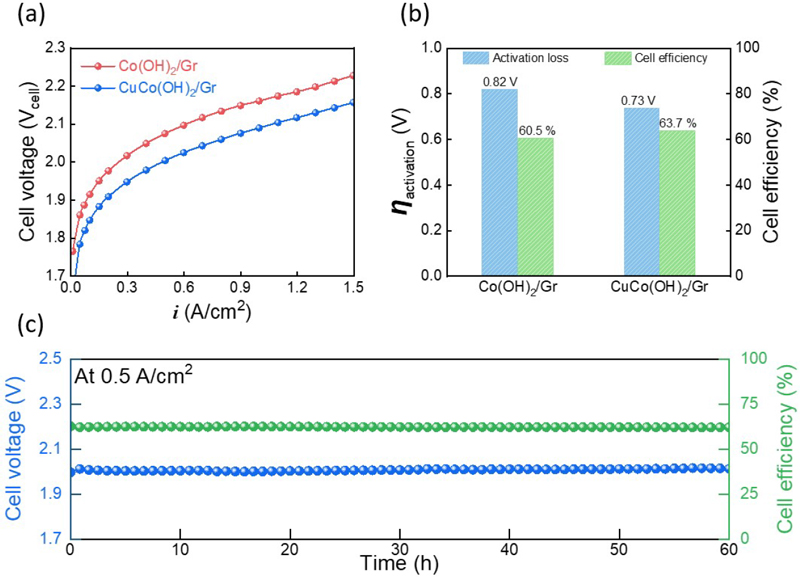
Polarization curves of the AEM electrolyzer were obtained to evaluate its performance. The AEM electrolyzer exhibited current densities of 0.3 A/cm2 and 0.5 A/cm2 at 2.0 Vcell when equipped with Co(OH)2/Gr and CuCo(OH)2/Gr, respectively (figure 4a). Given that all other conditions were identical except for the HER electrode, the difference in cell performance can be attributed to this electrode. Among the three voltage losses of the AEM electrolyzer (i.e., ohmic, activation, and mass transport losses), the activation loss has a direct correlation with the electrode performance. Therefore, the activation loss of the AEM electrolyzer at 0.5 A/cm2 was investigated; the results are depicted in Figure 4b. The activation loss of CuCo(OH)2/Gr was calculated at 0.73 V, which was 0.09 V lower than that of Co(OH)2/Gr. The energy-conversion efficiency of the AEM electrolyzer was then calculated (Figure 4b). The AEM electrolyzer equipped with CuCo(OH)₂/Gr demonstrated approximately 3.2% higher efficiency compared to that equipped with Co(OH)₂/Gr. The durability test of the AEM electrolyzer equipped with CuCo(OH)2/Gr was performed at 0.5 A/cm2 for 60 h, and the cell efficiencies throughout the test were calculated (Figure 4c). The cell voltage exhibits stable changes throughout the durability tests, with a degradation rate of 0.3 mV/h. In addition, the cell efficiency dropped only 1.49%, indicating that the AEM electro lyzer equipped with CuCo(OH)2/Gr demonstrated good durability.
4. Conclusion
We prepared CuCo(OH)2/Gr via electrodeposition and examined its utilization as an HER electrode for AEM electrolysis. The synthesized CuCo(OH)2 exhibits a fine nanosheet shape and yields a large surface area for electrochemical reactions, thereby attaining enhanced extrinsic activity. The incorporation of Cu into Co(OH)2 modifies the electronic structure of Co, enhancing its intrinsic activity. The AEM electrolysis cell equipped with CuCo(OH)2/Gr demonstrated higher efficiency than that with Co(OH)2/Gr.
Acknowledgments
This study was conducted with support from the Nano-infra Process Service Capability Enhancement Project (RS-2023-00258445) funded by the government (Ministry of Trade, Industry and Energy) in 2023.
Author Contributions
Conceptualization, Y. S. Park; Formal Analysis, I. T. Kim, J-M. Yeon, Investigation, Y. S. Park, S. -Y. Choi; Resources, S. -Y. Choi, J. -M. Yeon; Data Curation I. T. Kim and Y. S. Park; Writing-Original Draft Preparation, I. T. Kim; Writing-Review & Editing, Y. S. Park; Supervision, Y. S. Park; Funding Acquisition, Y. S. Park.
References
-
A. Mehtab, S. A. Ali, I. Sadiq, S. Shaheen, H. Khan, M. Fazil, N. A. Pandit, F. Naaz, and T. Ahmad, “Hydrogen energy as sustainable energy resource for carbon-neutrality realization,” ACS Sustainable Resource Management, vol. 1, no. 4, pp. 604-620, 2024.
[https://doi.org/10.1021/acssusresmgt.4c00039]

-
S. Shiva Kumar and H. Lim, “An overview of water electrolysis technologies for green hydrogen production,” Energy Reports, vol. 8, pp. 13793-13813, 2022.
[https://doi.org/10.1016/j.egyr.2022.10.127]

-
I. T. Kim, S. -H. Kim, J. S. Ha, T. H. Kim, J. Cho, G. D. Park, and Y. S. Park, “Oxygen deficient yolk–shell structured Co3O4 microspheres as an oxygen evolution reaction electrocatalyst for anion exchange membrane water electrolyzers,” Journal of Materials Chemistry A, vol. 11, pp. 16578-16585, 2023.
[https://doi.org/10.1039/D3TA02710D]

-
J. E. Park, S. Y. Kang, S. -H. Oh, J. K. Kim, M. S. Lim, C. -Y. Ahn, Y. -H. Cho, and Y. -E. Sung, “High-performance anion-exchange membrane water electrolysis,” Electrochimica Acta, vol. 295, pp. 99-106, 2019.
[https://doi.org/10.1016/j.electacta.2018.10.143]

-
S. Li, T. Liu, W. Zhang, M. Wang, H. Zhang, C. Qin, L. Zhang, Y. Chen, S. Jiang, D. Liu, X. Liu, H. Wang, Q. Luo, T. Ding, and T. Yao, “Highly efficient anion exchange membrane water electrolyzers via chromium-doped amorphous electrocatalysts,” Nature Communications, vol. 15, p. 3416, 2024.
[https://doi.org/10.1038/s41467-024-47736-0]

-
N. Du, C. Roy, R. Peach, M. Turnbull, S. Thiele, and C. Bock, “Anion-exchange membrane water electrolyzers,” Chemical Reviews, vol. 122, pp. 11830-11895, 2022.
[https://doi.org/10.1021/acs.chemrev.1c00854]

-
S. J. Lee, S. H. Lee, J. S. Ha, I. T. Kim, S. H. Park, H. K. Park, H. J. Park, B. K. Kang, and Y. S. Park, “Highly active cobalt–copper–selenide electrocatalysts for solar-driven oxygen evolution reaction: an electrochemical activation energy aspect,” ACS Sustainable Chemistry & Engineering, vol. 12, no. 1, pp. 275-282, 2024.
[https://doi.org/10.1021/acssuschemeng.3c05576]

-
A. Y. Faid, L. Xie, A. O. Barnett, F. Seland, D. Kirk, and S. Sunde, “Effect of anion exchange ionomer content on electrode performance in AEM water electrolysis,” International Journal of Hydrogen Energy, vol. 45, no. 53, pp. 28272-28284, 2020.
[https://doi.org/10.1016/j.ijhydene.2020.07.202]

-
G. Jerkiewicz, “Standard and reversible hydrogen electrodes: Theory, design, operation, and applications,” ACS Catalysis, vol. 10, no. 15, pp. 8409-8417, 2020.
[https://doi.org/10.1021/acscatal.0c02046]

-
Z. -Y. Liu, Q. -Y. Wang, and J. -M. Hu, “Introducing carbon dots to NiFe LDH via a mild coprecipitation–aging method to construct a heterojunction for effective oxygen evolution,” Catalysis Science & Technology, vol. 14, pp. 110-118, 2024.
[https://doi.org/10.1039/D3CY01621H]

-
L. A. King, M. A. Hubert, C. Capuano, J. Manco, N. Danilovic, E. Valle, T. R. Hellstern, K. Ayers, and T. F. Jaramillo, “A non-precious metal hydrogen catalyst in a commercial polymer electrolyte membrane electrolyser,” Nature Nanotechnology, vol. 14, pp. 1071-1074, 2019.
[https://doi.org/10.1038/s41565-019-0550-7]

-
K. Xu, P. Chen, X. Li, Y. Tong, H. Ding, X. Wu, W. Chu, Z. Peng, C. Wu, and Y. Xie, “Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation,” Journal of the American Chemical Society, vol. 137, pp. 4119-4125, 2015.
[https://doi.org/10.1021/ja5119495]

-
B. Cao, C. Luo, J. Lao, H. Chen, R. Qi, H. Lin, and H. Peng, “Facile synthesis of 3d transition-metal-doped α-co(oh)2 nanomaterials in water–methanol mediated with ammonia for oxygen evolution reaction,” ACS Omega, vol. 4, pp. 16612-16618, 2019.
[https://doi.org/10.1021/acsomega.9b02504]

-
Y. Xu, C. Li, W. Mei, M. Guo, Y. Yang, “Equivalent circuit models for a biomembrane impedance sensor and analysis of electrochemical impedance spectra based on support vector regression,” Medical & Biological Engineering & Computing, vol. 57, pp. 1515-1524, 2019.
[https://doi.org/10.1007/s11517-019-01970-7]