Study on behavior and emission of methane/hydrogen diffusion flame with applied DC electric field
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This experimental study investigates the effect of DC electric fields on flame behavior and emissions in non-premixed hydrogen/methane flames using a co-flow burner. The study considers varying hydrogen mole fractions of 0.1, 0.3, and 0.5, and different fuel flow velocities of 2.0, 6.3, and 8.0 cm/s. To introduce DC electric fields, a high voltage terminal was connected to the fuel nozzle, while the ground terminal was connected to an upper mesh located above the flame. The applied DC voltages ranged from Vdc = -10 kV to +10 kV. The study reveals that the addition of hydrogen reduces flame height. Furthermore, when subjected to DC electric fields, the flames exhibit movement towards the lower potential side, displaying stable and oscillating behaviors due to the Lorentz force acting on positive ions within the flames. The concentrations of CO and CO2 exhibit insignificant changes with varying hydrogen mole fractions. However, when exposed to positive voltage, CO, CO2, and NOX emissions are reduced. This research provides a detailed analysis of flame/flow behavior and emission characteristics when hydrogen and DC electric fields are introduced, providing valuable insights into the control of flame behavior and pollutant emissions.
Keywords:
Methane/hydrogen blended flame, Reduction of NOX, Co-flow burner, DC electric field, Stability of flame1. Introduction
Recently, the world has experienced a global crisis due to abnormal climates caused by global warming. To minimize domestic damage caused by the climate crisis and contribute to international efforts to address the climate crisis, the Republic of Korea has declared its commitment to achieving carbon neutrality by 2050. In this mitigation plan, the Republic of Korea attempts to reduce carbon emissions by discontinuing the use of fossil fuels for power generation and transitioning to zero-carbon fuels such as hydrogen and ammonia. By switching to zero-carbon fuels, the Republic of Korea intends to achieve carbon neutrality by 2050 [1]. The Republic of Korea also plans to apply technologies for transitioning from fossil to zero-carbon fuels in engines and boilers utilized in the transportation sector, which currently rely on combustion. Furthermore, in the domestic gas turbine sector, the Republic of Korea is promoting the transition to a hydrogen-based economy by blending hydrogen with fossil fuels. When hydrogen, a zero-carbon fuel, is mixed at a volume ratio of 50% with LNG, it can result in approximately a 23% reduction in CO2 emissions compared to conventional methods [2]. Currently, the Republic of Korea has set a National Greenhouse Gas Reduction Target (NDC) of a 40% reduction by 2030, based on the 2018 greenhouse gas emissions baseline. The transportation sector is planning to achieve a reduction of approximately 37.8% in greenhouse gas emissions [3]. To realize carbon neutrality, the use of combustion with zero-carbon fuels is indispensable.
Hydrogen exists in its lightest and least dense form among conventional gaseous fuels. Mixing hydrogen with methane accelerates flame propagation, widens the flammable range, and increases the risk of explosions. During hydrogen combustion, the post-combustion products result in a decrease in CO and CO2 while relatively increasing water vapor (H2O). However, hydrogen flames are characterized by the generation of thermal NOX owing to high temperatures [4]. Therefore, when utilizing hydrogen, reducing carbon emissions is essential; nevertheless, it is equally important to mitigate NOX emissions, a precursor to fine particulate matter. Hence, combustion techniques capable of reducing NOX emissions are necessary.
Previous studies have demonstrated that applying a DC electric field to hydrocarbon flames leads to flame stabilization [5], increased flame propagation speed, and reduced soot formation [6]-[7]. These effects are attributed to the ion wind generated when an electric field is applied to hydrocarbon flames [8]. During the combustion of hydrocarbons, chemi-ionization occurs at the flame front, generating positive ions, negative ions, and electrons [9]. When an electric field is applied, these ions are accelerated by the Lorentz force, resulting in the generation of an ion wind as they collide with neutral molecules. The generation of negative ions is approximately 10% that of positive ions, making the ion wind predominantly directed in the direction of positive ion movement [10]-[11].
In a co-flow jet diffusion flame, Kelvin-Helmholtz instability is known to occur owing to the shear layer between the fuel and oxidizer, and the oscillation typically has a frequency in the range of 10 to 20 Hz [12]-[13]. When an oscillatory flame is formed, combustion becomes unstable, resulting in a significant decrease in thermal efficiency, generation of noise, and structural damage to the combustion chamber due to pressure oscillations during the oscillation [14]. Therefore, extensive research is being conducted to control unstable flames within combustion chambers.
Here, experimental research was conducted to investigate flame behavior with hydrogen addition in methane diffusion flames and analyze flame behavior and exhaust gas trends when a DC electric field is applied to flames with Kelvin-Helmholtz instability.
2. Experimental Setup and Methods
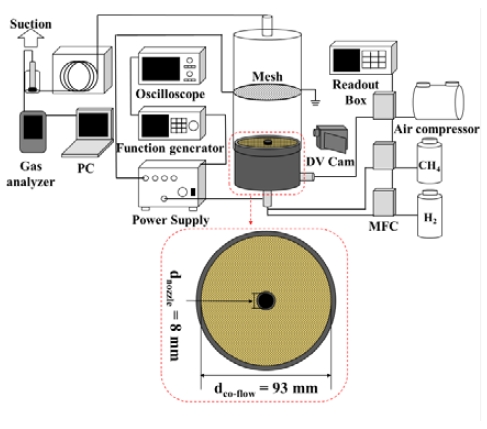
An experimental setup, as illustrated in Figure 1, was adopted to investigate changes in flame behavior and exhaust gas emissions with the addition of hydrogen and application of an electric field to methane/hydrogen diffusion flames. A co-flow burner was utilized to create diffusion flames, comprising a fuel nozzle with inner and outer diameters of 8 and 10 mm, respectively, and a co-flow burner body with an inner diameter of 93 mm. To apply an electric field to the flame, a high voltage was connected to the nozzle, and grounding was established at the tip of the nozzle, positioned 100 mm above it, using a metal mesh. Methane (99.9% purity) and hydrogen (99.9% purity) were supplied through the fuel nozzle, while air was utilized as the oxidizer and supplied externally to the fuel nozzle.
The experiments were conducted by varying the hydrogen mole fraction (XH2) within the fuel from 0.0 to 0.1, 0.3, and 0.5 in incremental steps, while maintaining constant fuel velocities (Uj) of 2.0 cm/s, 6.3 cm/s, and 8.0 cm/s. The air velocity (Uco-flow) was held constant at 5.3 cm/s. Flow control of the mixed fuel and air was achieved using a mass flow controller (MFC) from Atovec (AFC 500) together with a Readout box (Atovec, GMC1200). To apply the DC electric field, high voltages of ± 2 kV, ± 5 kV, and ± 10 kV were supplied using a power supply (Trek 10/10B-HS) and a function generator (Tektronix, AFG 1062). The applied voltages were monitored using an oscilloscope (Tektronix, MDO34). These experimental parameters allowed us to investigate the flame behavior and exhaust gas emissions under various conditions of hydrogen mole fraction, fuel velocity, and applied DC electric field.
To directly observe changes in flame behavior, a DV Cam (Sony, HQR-PJ675) was utilized. Flame observations were achieved by tracking variations in flame height, and Matlab was adopted to derive the flame height. Furthermore, the fast Fourier transform (FFT) was employed to determine the frequency and amplitude of oscillatory flames. To measure the exhaust gases generated during combustion, a chamber was installed above the burner. Gas concentrations of CO, CO2, and NOX in the exhaust gases were monitored using a gas analyzer (TESTO, 350 K) for a duration of 300 s, and the time in which the gas concentrations reached saturation was utilized as a reference point. The measured gas concentrations were corrected based on an assumed oxygen concentration of 16%. This setup allowed us to characterize the exhaust gas emissions resulting from the combustion process.
3. Result and Discussion
3.1 Effects of Hydrogen Addition on Methane Diffusion Flame
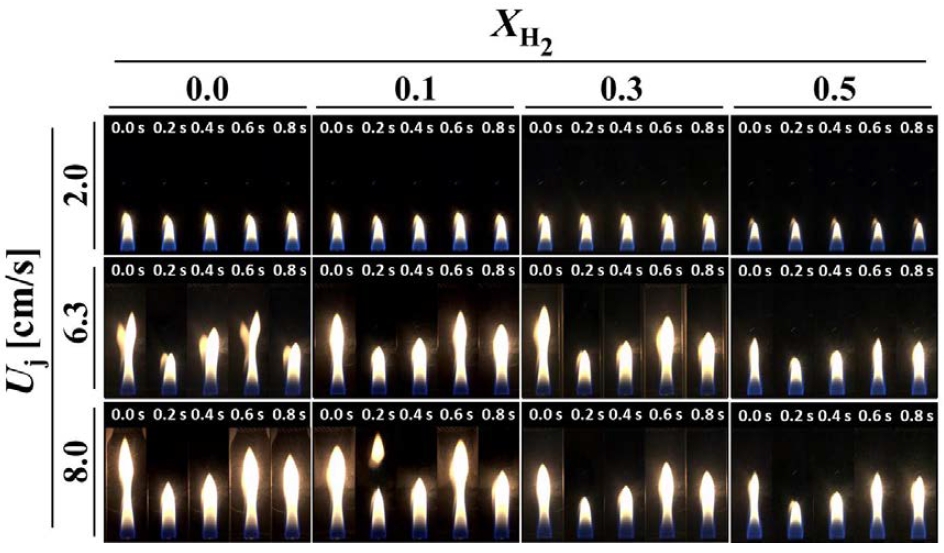
Figure 2 illustrates the changes in flame behavior over 0.8 s due to variations in flow velocity and hydrogen addition to methane diffusion flames. For pure methane flames (XH2 = 0.0) at a fuel velocity of 2.0 cm/s, a typical co-flow jet flame is observed without oscillations. When the hydrogen mole fraction is increased from 0.0 to 0.3, there is no significant visual change in the flame. However, at XH2 = 0.5, a reduction in flame length can be observed. This reduction agrees well with Roper's theory, which predicts that flame length decreases with changes in density, volume, and velocity within the flame [15]. At fuel velocities of 6.3 cm/s and 8.0 cm/s for methane flames (XH2 = 0.0), Kelvin-Helmholtz instability oscillations due to the shear layer in the combustion flow fields are observed. The amplitude of these oscillations increases with higher fuel velocities. For XH2 = 0.1 and XH2 = 0.3, there are no significant differences in flame behavior compared to methane diffusion flames without hydrogen addition. However, at XH2 = 0.5, the flame amplitude decreases, and the reduction in the carbon content of the fuel suppresses the formation of soot particles, thereby diminishing the yellow luminous region. This decrease in flame amplitude with increasing hydrogen mole fraction is attributed to the high reactivity of hydrogen fuel. Notably, the addition of hydrogen stabilizes the oscillating flame, leading to the formation of a more stable flame.
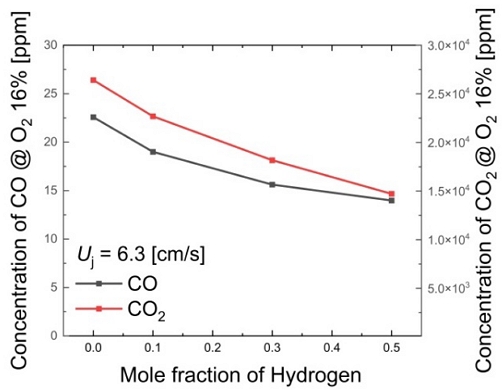
At a fuel velocity of 6.3 cm/s, Figure 3 presents the trends in CO and CO2 emissions with hydrogen addition. As the hydrogen mole fraction increases, it is evident that both CO and CO2 emissions decrease. When the hydrogen mole fraction is increased to 0.5, compared to pure methane flames (XH2 = 0.0), there is approximately a 38.1% reduction in CO emissions and an estimated 44.3% reduction in CO2 emissions. This reduction agrees with the decrease in the carbon content within the fuel as hydrogen mole fraction increases, including the suppression of soot particle formation, resulting in a decrease in the yellow luminous region, as observed in the changes in flame behavior. This implies that the combustion of hydrogen fuel leads to a reduction in carbon emissions in exhaust gases.
3.2 Effects of DC Electric Field on Methane/Hydrogen Diffusion Flame
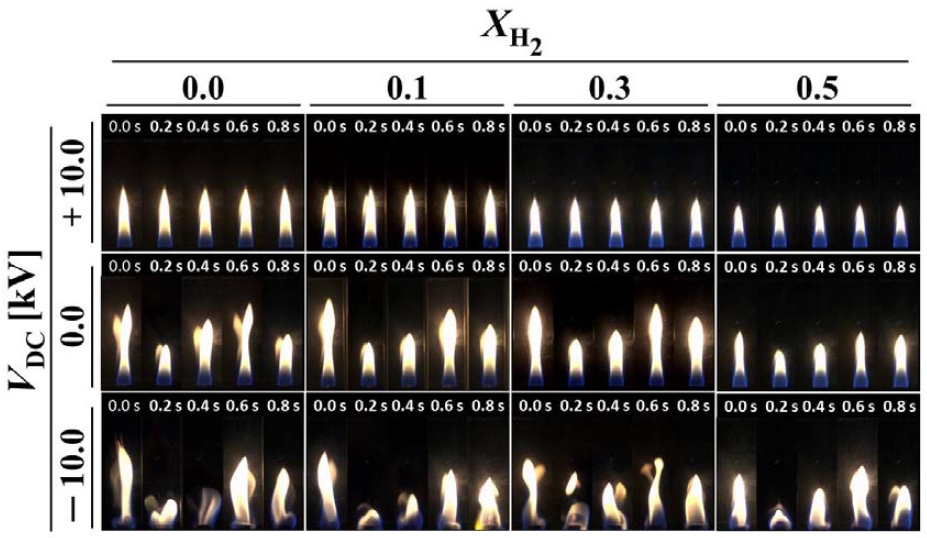
Figure 4 illustrates the changes in flame behavior with varying hydrogen mole fractions and the application of an electric field in methane/hydrogen diffusion flames where Kelvin-Helmholtz (K-H) instability has been formed (Uj = 6.3 cm/s). When no electric field is applied, the direct photographs of the flames with hydrogen addition are the same as those in Figure 2.
The flame length decreases with an increase in the hydrogen mole fraction. Regardless of the hydrogen mole fraction, when a positive voltage is applied, it can be observed that K-H instability forms in all flames. When Vdc = +10 kV is applied, stable flames are observed in all cases, and oscillations are absent. The stabilization of flames is likely owing to the influence of ion wind generated at the flame front, which affects the shear layer between the fuel and air, thereby reducing instability.
However, when a negative voltage (Vdc = -10 kV) is applied, all flames become unstable, regardless of the hydrogen addition. Ions generated within the flame are likely to move towards the nozzle, and the ion wind acts in the opposite direction to +10 kV, thus accelerating flame instability. By directly applying a DC electric field to the flames, it is possible to control flame instability, as confirmed by these results.
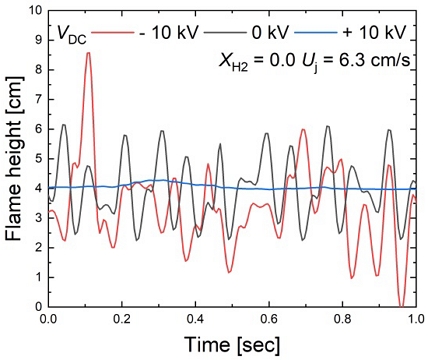
Figure 5 presents the flame heights of flames with and without the application of an electric field in methane flames (XH2 = 0.0), where ±3.39 cm of oscillation is observed. When no electric field is applied, a flame with an average height of 3.90 cm exhibits oscillations with a ±1.78-cm amplitude and consistent frequency.
However, when a +10-kV voltage is applied, the flame height remains at 4.00 cm without oscillations. Conversely, when a -10-kV voltage is applied, an unstable flame is observed with an average height and amplitude of 3.40 cm and ±3.39 cm, respectively.
This study focused on investigating flames subjected to positive voltage input, which exhibited stable flames with a consistent frequency, rather than fully unstable flames caused by negative voltage input.
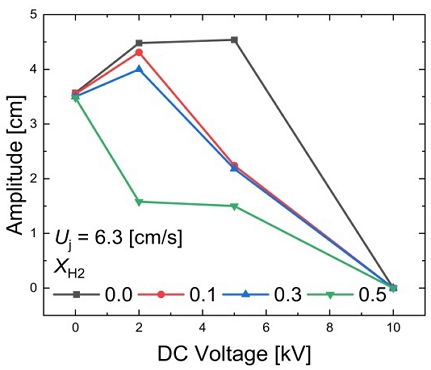
The amplitude variations relative to the applied voltage magnitude in methane/hydrogen diffusion flames at Uj = 6.3 cm/s are illustrated in Figure 6. In methane diffusion flames, it is evident that the amplitude initially increases with the magnitude of the applied voltage, and then disappears at +10 kV. Remarkably, for XH2 = 0.5, the amplitude consistently decreases with increasing positive voltage magnitude. However, for hydrogen mole fractions less than 0.5, there is a tendency for the amplitude to increase slightly with increasing voltage magnitude before decreasing.
These observations suggest that further chemical and physical analysis may be required to elucidate the effects of hydrogen addition on the ion formation via chemi-ionization at the flame front. The complex relationship between the hydrogen mole fraction, voltage magnitude, and flame dynamics requires more in-depth investigation.
Table 1 presents the oscillation frequencies of the flames analyzed via FFT in methane/hydrogen flames with varying applied electric field strengths. When no electric field is applied to the flames, the flame frequency oscillates at approximately 11.6 Hz. The flame frequency tends to increase to approximately 12.6 Hz as the applied positive voltage tends to +5 kV. Moreover, this trend indicates that the oscillation frequency remains relatively constant when the same magnitude of positive voltage is applied, regardless of the hydrogen mole fraction. Such frequencies correspond to the conditions under which Kelvin-Helmholtz instability forms [12]. However, at +10 kV, a stable flame is formed without oscillations. As aforementioned, it is assumed that at +10 kV, the ion wind generated from the flame to the electrode affects the shear layer, thus stabilizing the oscillating flame. To verify this assumption, further three-dimensional analysis is required, and simulation studies are planned for the future.
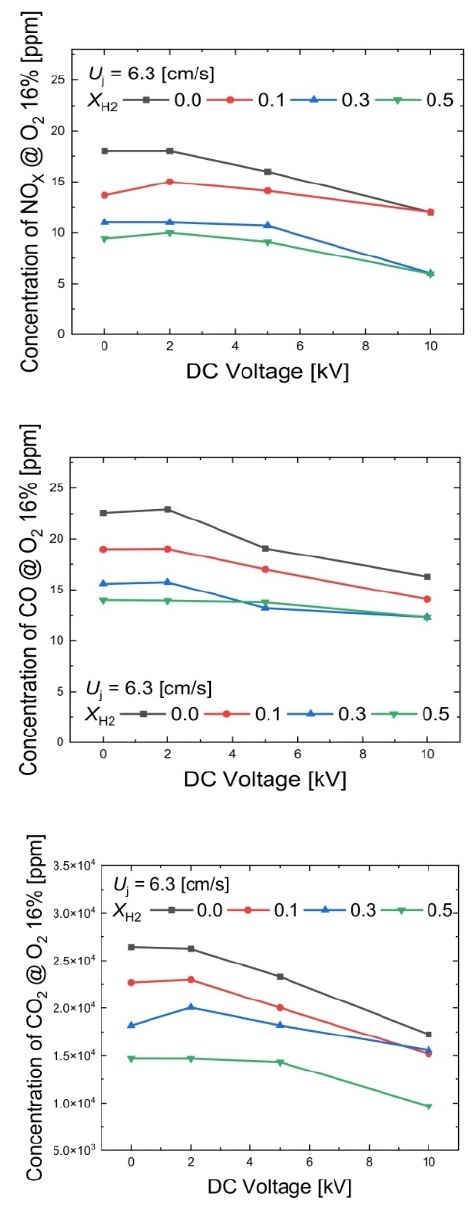
Figure 7 illustrates the changes in emission trends in the presence of an applied voltage in methane/hydrogen diffusion flames at Uj = 6.3 cm/s. When no electric field is applied, it can be observed that as the hydrogen mole fraction increases, CO, CO2, and NOX emissions decrease. This decrease in CO and CO2 was mentioned in Section 3.1, where the increase in hydrogen, a carbon-free fuel, leads to reduced CO and CO2 emissions. Regarding NOX, it is inferred that the heating value of the fuel decreases with an increase in the hydrogen mole fraction, which results in a reduction in flame temperature and subsequently NOX emissions.
As the voltage magnitude increases, it is evident that CO, CO2, and NOX emissions decrease. When an electric field is applied, the stabilization of the flame and suppression of soot formation lead to reductions in CO and CO2 emissions. For NOX, the increase in the chemical reaction zone due to ion wind leads to a decrease in NOX emissions [16]-[19]. To interpret these results more clearly, it would be necessary to examine the changes in chemical species. When +10-kV voltage is applied to the methane (XH2 = 0.0) diffusion flame at a fuel velocity of 6.3 cm/s, CO, CO2, and NOX emissions decrease by 27.8%, 34.8%, and 33.2%, respectively. When +10-kV voltage is applied with a hydrogen mole fraction of 0.5, CO, CO2, and NOX emissions decrease by 11.8%, 34.3%, and 36.9%, respectively. Although the changes in CO and CO2 emissions decrease as the hydrogen mole fraction increases, the change in NOX emissions increases.
In hydrocarbon combustion flames, the initial stage involves reactions such as CH + O → CHO+ + e-, leading to the formation of ions. It was anticipated that an increase in the hydrogen mole fraction would lead to a reduction in the number of ions in the flame, thus diminishing the impact of the electric field. However, the reduction in NOX emissions was even more significant. To provide a clear interpretation of this phenomenon, it is crucial to investigate the changes in chemical species within the flame. In general, the application of a DC electric field to the flame effectively controls exhaust pollutants. Future research is required for its practical implementation in combustion systems.
4. Conclusion
The investigation of the effects of hydrogen addition and the application of a DC electric field on methane/hydrogen diffusion flames has yielded the following conclusions:
- 1. The introduction of hydrogen to methane diffusion flames, particularly at a hydrogen mole fraction of 0.5, resulted in reduced flame oscillation amplitudes. This reduction is attributed to the high reactivity of hydrogen, which leads to the formation of more stable flames. The decrease in carbon content within the fuel due to hydrogen addition also contributed to the suppression of soot particle formation, which diminished the yellow luminous region. Consequently, there was a significant reduction in the emissions of CO (38.1% decrease) and CO2 (44.3% decrease).
- 2. When a DC electric field was applied to methane/hydrogen diffusion flames with a fuel velocity of 6.3 cm/s, flame oscillations were observed at frequencies ranging from 11.6 to 12.6 Hz, indicative of Kelvin-Helmholtz instability. However, when a +10-kV voltage was applied, these oscillations disappeared, suggesting flame stabilization. The amplitude of the oscillating flame increased with the applied voltage, reaching its peak before disappearing at +10 kV. This effect is attributed to the formation of ion-induced airflow due to chemi-ionization, which interacts with the shear layer between the fuel and air to stabilize the flame. Further chemical-physical analysis is required for a comprehensive understanding.
- 3. The addition of hydrogen and the application of an electric field resulted in reduced concentrations of CO, CO2, and NOX in methane flames. The decrease in CO and CO2 concentrations is attributed to the reduction in carbon content within the fuel and flame stabilization due to the electric field, which led to reduced soot formation and emissions. Regarding NOX, the addition of hydrogen reduced the flame's heat release rate, which decreased the flame temperature and subsequently reduced NOX emissions. The application of an electric field increased the chemical reaction region within the flame, further reducing NOX emissions. Although the changes in CO and CO2 emissions decreased with increasing hydrogen mole fractions, NOX emissions exhibited an increasing trend. A detailed analysis of chemical species is required for a precise explanation.
These findings indicate that hydrogen addition and the application of a DC electric field can effectively control flame characteristics and pollutant emissions in methane-based diffusion flames, which is promising for practical implementations in combustion systems.
Acknowledgments
This research was supported by the National Research Council of Science & Technology (NIST) grant by the Korea government (MIST) (No. CPS22071-100).
Author Contributions
Conceptualization; D. G. Park and S. H. Yoon, Writing—Review & Editing; D. G. Park, Writing—Original Draft Preparation; B. H. Seok, Investigation; B. H. Seok and J. S. Kim, Data Curation; B. H. Seok and J. S. Kim, Methodology; D. G. Park, S. H. Yoon, B. H. Seok, J. S. Kim, and J. Hwang, Visualization; D. G. Park, B. H. Seok, and J. S. Kim, Software; B. H. Seok, J. Hwang, Supervision; D. G. Park, Funding Acquisition; D. G. Park.
References
- The Ministry of Environment, 2050 Carbon Neutral Scenario, Korea, 2021.
- KOSPO, Development of hydrogen mixing technology for large gas turbines, Korea, 2023.
- The Ministry of Environment, The Republic of Korea’s Enhanced Update of its First Nationally Determined Contribution, Korea, 2021.
-
J. Park, J. S. Park, J. S. Kim, S. C. Kim, S. I. Keel, J. H. Yun, and W. H. Kim, “A study on effects of hydrogen addition in methane-air diffusion flame,” Transactions of the Korean Society of Mechanical Engineers - B, vol. 31, no. 4, pp. 384-391, 2007.
[https://doi.org/10.3795/KSME-B.2007.31.4.384]

-
S. M. Lee, C. S. Park, M. S. Cha, and S. H. Chung, “Effect of electric fields on the liftoff of nonpremixed turbulent jet flames,” IEEE Transactions on Plasma Science, vol. 33, no. 5, pp. 1703-1708, 2005.
[https://doi.org/10.1109/TPS.2005.856414]

-
M. S. Cha and Y. Lee, “Premixed combustion under electric field in a constant volume chamber,” IEEE Transactions on Plasma Science, vol. 40, no. 12, pp. 3131-3138, 2012.
[https://doi.org/10.1109/TPS.2012.2206120]

-
M. Saito, T. Arai, and M. Arai, “Control of soot emitted from acetylene diffusion flames by applying an electric field,” Combustion and Flame, vol. 119, no. 3, pp. 356-366, 1999.
[https://doi.org/10.1016/S0010-2180(99)00065-6]

-
M. K. Kim, S. K. Ryu, S. H. Won, and S. H. Chung, “Electric fields effect on liftoff and blowoff of nonpremixed laminar jet flames in a coflow,” Combustion and Flame, vol. 157, no. 1, pp. 17–24, 2010.
[https://doi.org/10.1016/j.combustflame.2009.10.002]

-
D. G. Park, B. C. Choi, M. S. Cha, and S. H. Chung, “Soot reduction under DC electric fields in counterflow non-premixed laminar ethylene flames,” Combustion Science and Technology, vol. 186, pp. 644-656, 2014.
[https://doi.org/10.1080/00102202.2014.883794]

-
D. G. Park, S. H. Chung, and M. S. Cha, “Bidirectional ionic wind in nonpremixed counterflow flames with DC electric fields,” Combustion and Flame, vol. 168, pp. 138-146, 2016.
[https://doi.org/10.1016/j.combustflame.2016.03.025]

-
D. G. Park, S. H. Chung, and M. S. Cha, “Visualization of ionic wind in laminar jet flames,” Combustion and Flame, vol. 184, pp. 246-248, 2017.
[https://doi.org/10.1016/j.combustflame.2017.06.011]

-
I. Kimura, “Stability of laminar-jet flame,” 10th Symposium on Combustion, vol. 10, pp. 1295-1300, 1965.
[https://doi.org/10.1016/S0082-0784(65)80264-8]

-
H. Yoshikawa and J. Wesfreid, “Oscillatory Kelvin–Helmholtz instability. Part 1. A viscous theory,” Journal of Fluid Mechanics, vol. 675, pp. 223-248, 2011.
[https://doi.org/10.1017/S0022112011000140]

-
T. C. Lieuwen, V. Yang, “Combustion instabilities in gas turbine engines: Operational experience, fundamental mechanisms, and modeling,” the American Institute of Aeronautics and Astronautics, 2005.
[https://doi.org/10.2514/4.866807]

-
F. G. Roper, “The prediction of laminar jet diffusion flame sizes: Part 1. Theoretical model,” Combustion and Flame, vol. 29, pp. 219-226, 1977.
[https://doi.org/10.1016/0010-2180(77)90112-2]

-
P. H. Joo, Y. Wang, A. Raj, and S. H. Chung, “Sooting limit in counterflow diffusion flames of ethylene/propane fuels and implication to threshold soot index,” Proceedings of the Combustion Institute, vol. 34, no. 1, p. 1803, 2013.
[https://doi.org/10.1016/j.proci.2012.06.124]

-
T. Rutar, D. C. Horning, J. C. Y. Lee, and P. C. Malte, “NOx dependency on residence time and inlet temperature for lean-premixed combustion in jet-stirred reactors,” Proceedings of the ASME 1998 International Gas Turbine and Aeroengine Congress and Exhibition, Volume 3: Coal, Biomass and Alternative Fuels; Combustion and Fuels; Oil and Gas Applications; Cycle Innovations. Stockholm, Sweden, 1998.
[https://doi.org/10.1115/98-GT-433]

- Y. N. Chun and D. Y. Shin, “Reduction of NOx emission from fuel nitrogen in new staged fueling system (1) (Characteristics of NOX formation & reduction)”, Journal of Korean Society for Atmospheric Environment, vol. 10, no. E, pp. 303-310, 1994.
-
G. Wang, T. F. Guiberti, S. Cardona, C. Avila Jimenez, and W. L. Roberts, “Effects of residence time on the NOx emissions of premixed ammonia-methane-air swirling flames at elevated pressure,” Proceedings of the Combustion Institute, vol. 39, no. 4, pp. 4277-4288, 2023.
[https://doi.org/10.1016/j.proci.2022.07.141]