Separation of dissolved gases from water using synthesized gases based on exhalation characteristics
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
It’s possible for a human to breathe under water, if dissolved oxygen is effectively used. Fish can stay under water using the gill which extracts dissolved oxygen from water. Water includes small amounts of oxygen, so a human needs larger amounts of water to acquire oxygen enough for underwater breathing. The exhalation gas from a human is another method to get higher amounts of oxygen under water. It mainly composes of oxygen, nitrogen and carbon dioxide. So, if only carbon dioxide is decreased, the exhalation gas has good characteristics for breathing of a human under water.
In this paper, composition of the exhalation gas from a human was analyzed using GC. Based on these results, the synthesized gas was prepared and mixed into water which was used for experimental devices to analyze separation characteristics of dissolved gases from water. Experimental devices included a water pump, a hollow fiber membrane module and a vacuum pump. The effects of pressure and water flow on separation characteristics of synthesized gas were investigated. The compositions of gases separated from water using synthesized gas were investigated using GC. These results expect to be applied to the development of underwater breathing technology for a human.
Keywords:
Exhalation, Synthesized gases, Underwater, Separation1. Introduction
Dissolved oxygen can make corrosion in the pipe and reduce operating time. To solve the problem, effective removal of dissolved oxygen is needed. Hollow fiber membrane modules are generally used due to high surface area per unit volume [1].
It’s possible for a human to breathe under water, if dissolved oxygen is effectively used. Fish can stay under water using the gill which extracts dissolved oxygen from water. A fish has effective extraction structure[2]. Water includes small amounts of oxygen, so a human needs larger amounts of water to acquire oxygen enough for underwater breathing. Dissolve oxygen is used to find the possibility of underwater breathing for a dog [3]. To get higher amounts of oxygen, effective methods are need [4]-[7].
The exhalation gas from a human is another method to get higher amounts of oxygen under water. It mainly composes of oxygen, nitrogen and carbon dioxide. So, if only carbon dioxide is decreased, the exhalation gas has good characteristics for breathing of a human under water.
In this paper, composition of the exhalation gas from a human was analyzed using GC. Based on these results, the synthesized gas was prepared and mixed into water which was used for experimental devices to analyze separation characteristics of dissolved gases from water. Experimental devices included a water pump, a hollow fiber membrane module and a vacuum pump. The effects of pressure and water flow on separation characteristics of synthesized gas were investigated. The compositions of gases separated from water using synthesized gas were investigated using GC. These results expect to be applied to the development of underwater breathing technology for a human.
2. Experimental
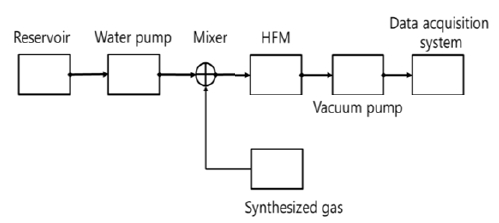
Figure 1 shows the block diagram for experimental devices. The synthesized gas prepared on basis of the exhalation gas sampled from a human is used to analyze the effects of flow rates of exhalation gas and water on composition of gases separated from water. A hollow fiber membrane module is from the Liqui-Cell company. Its specifications are represented in Table 1.
3. Resultsand Discussions
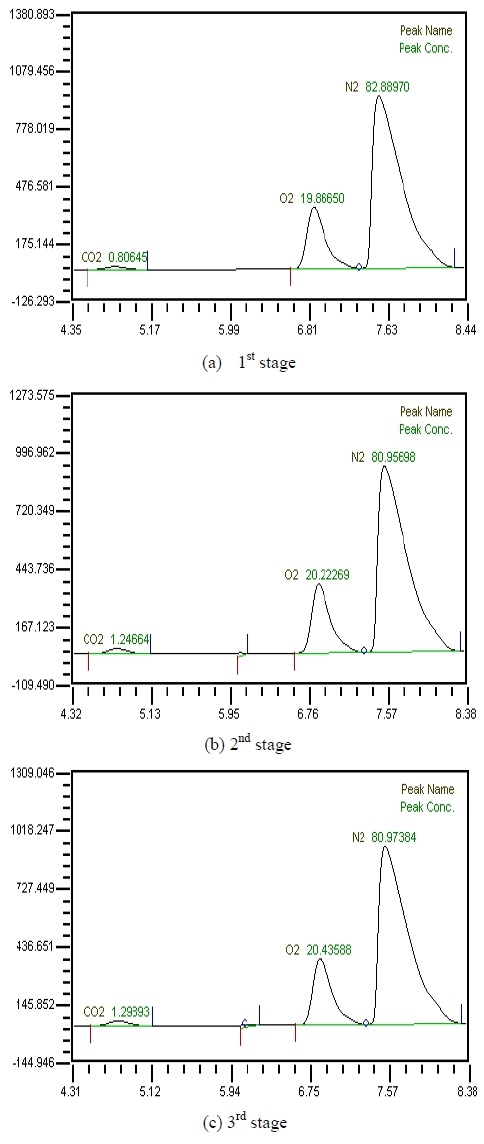
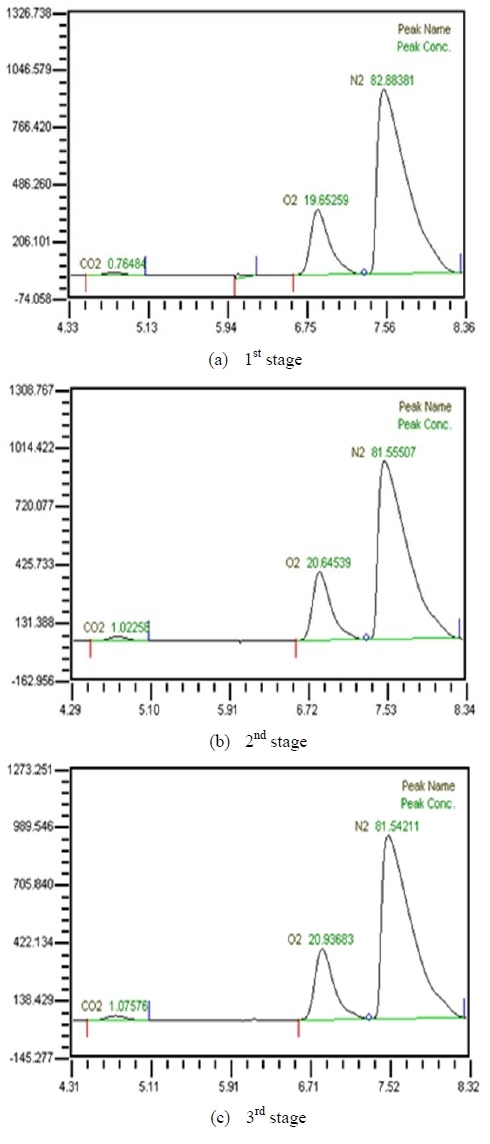
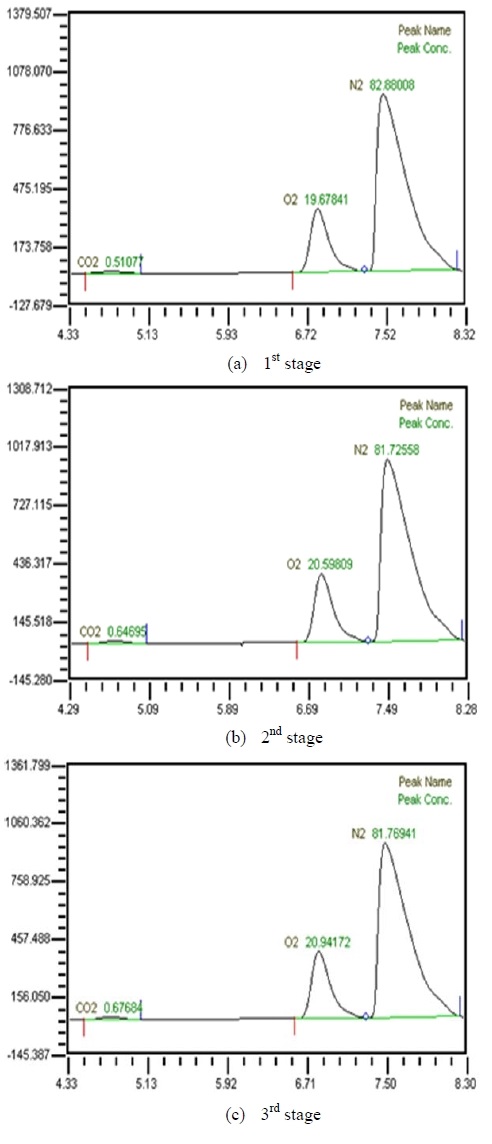
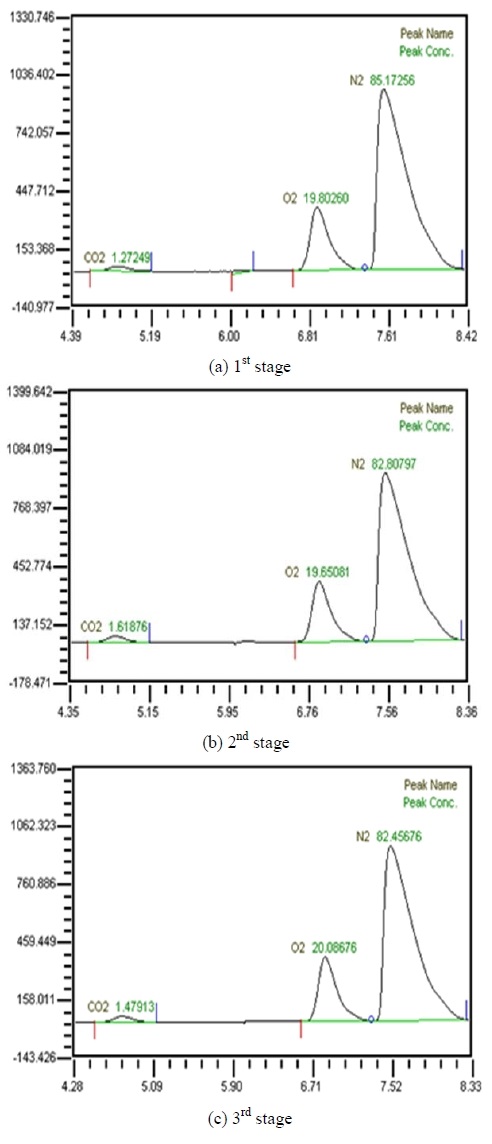
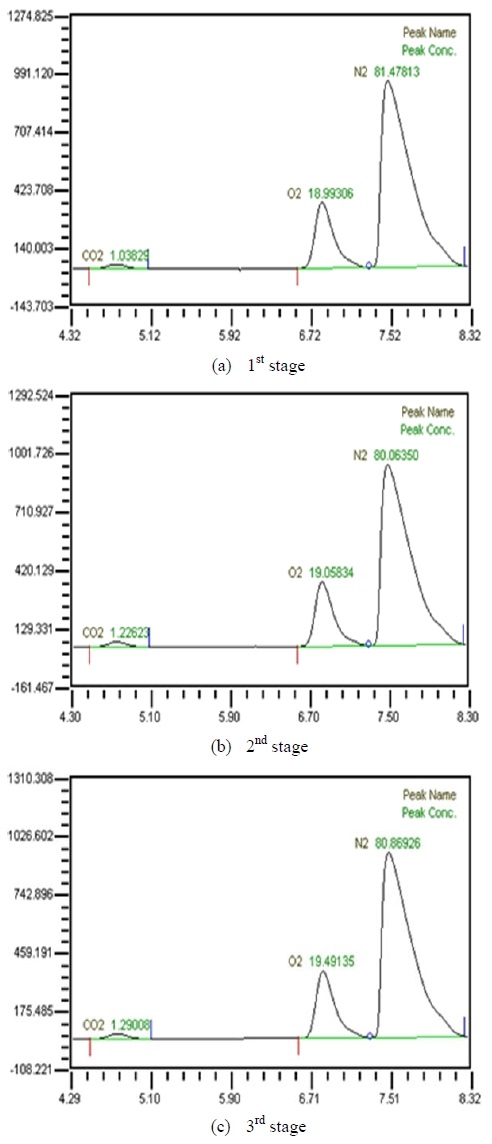
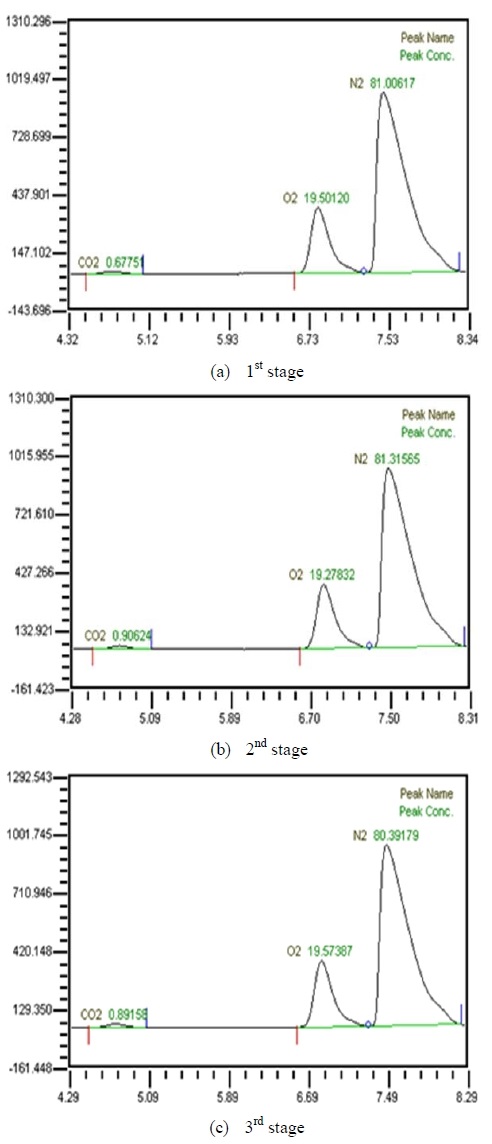
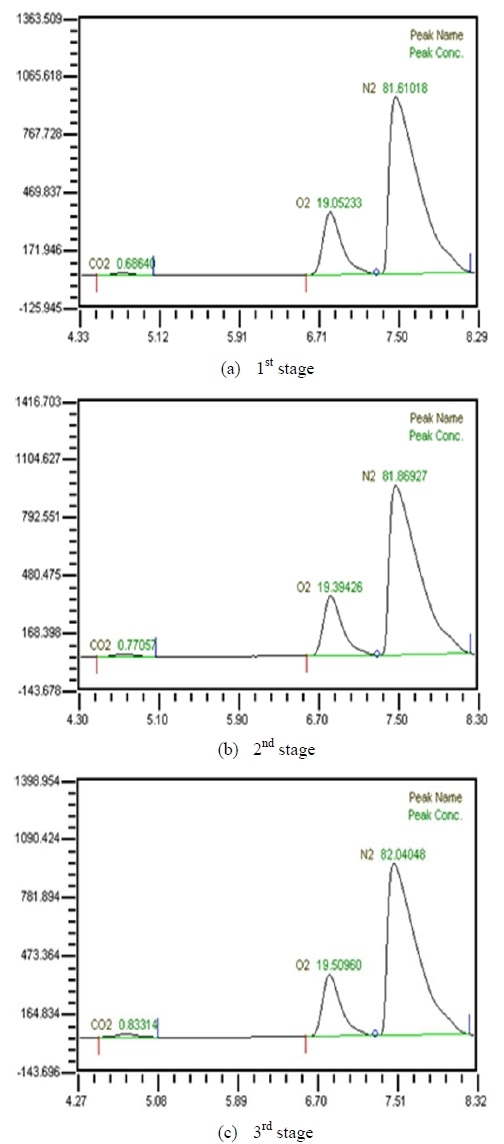
Table 2 shows the composition of synthesized gases based on the measurement of composition of gases sampled for a human. A home-made vacuum pump is used to make 3 kinds of vacuum in the lumen side, 1st, 2nd and 3rd stage. 1st stage means no vacuum, 2nd stage 50% of the max vacuum and 3rd stage 60% of the max vacuum. Synthesized gases were supplied at 1, 2, 3 LPM and water at 10, 20, 30 and 40 LPM. Figure 2, 3, 4 and 5 show measurements from sampled bag using GC with 1 LPM of synthesized gas and 10, 20, 30 and 40 LPM of water flow. The amounts of carbon dioxide separated from water were much less than initial state of exhalation. As the vacuum state was increased, the amounts of carbon dioxide were increased. Figure 6, 7, 8 and 9 show measurements from sampled bag using GC with 2 LPM of synthesized gas and 10, 20, 30 and 40 LPM of water flow. As vacuum state was increased, the amounts of carbon dioxide were increased. As water flow rates were increased, the amounts of carbon dioxide were decreased.
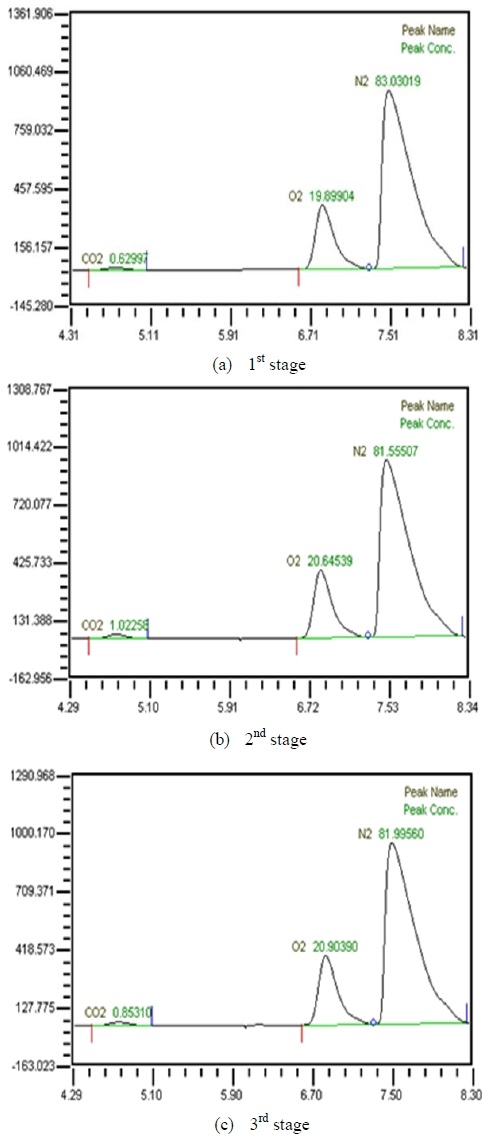
Table 3 shows composition of gases separated from water with 3 LPM exhalation gas. 10, 20, 30 and 40 LPM of water were supplied using a water pump. As the flow rates of synthesized gas were increased, the amounts of carbon dioxide were increased. As water flow rates were increased, the amounts of carbon dioxide were decreased. At flow rates of 3 LPM of synthesized gas and 40 LPM of water, the amounts of carbon dioxide represented 0.88 %. So this level of carbon dioxide can be used in underwater breathing. And oxygen separated through the membrane module from mixed state can be also used. These results mean that pretreated exhalation gases can be used in underwater breathing.
4. Conclusions
We investigated composition of exhalation gas separated from water with variations of flow rates of synthesized gas and water. As the vacuum state was increased, the amounts of carbon dioxide were increased. As water flow was increased, the amounts of carbon dioxide were decreased. On the condition of 3 LPM of synthesized gas and 40 LPM of water flow, 0.88 % of carbon dioxide was measured from separated gas using GC. This means pretreated exhalation gas can be used in underwater breathing. These results expect to be applied to the enhancement of separating efficiency of an artificial gill system.
Acknowledgments
This paper is extended and updated from the short version that appeared in the Proceedings of the International symposium on Marine Engineering and Technology (ISMT 2014), held at Paradise Hotel, Busan, Korea on September 17-19, 2014.
References
-
X. Tan, G. Capar, and K. Li, “Analysis of dissolved oxygen removal in hollow fibre membrane modules: effect of water vapour”, Journal of Membrane Science 251, p111-119, (2005).
[https://doi.org/10.1016/j.memsci.2004.11.005]

-
K. Park, W. Kim, and H. Y. Kim, “Optimal lamellar arrangement in fish gills”, Proceedings of the National Academy of Sciences of the United States of America, 111(22), p8067-8070, (2014).
[https://doi.org/10.1073/pnas.1403621111]

-
M. Yang, and E. L. Cussler, “Artificial gills”, Journal of Membrane Science 42, p273-284, (1989).
[https://doi.org/10.1016/S0376-7388(00)82381-9]

-
K. Nagase, U. Hasegawa, and F. Kohori, “The photoresponse of a molybdenum porphyrin makes an artificial gill feasible”, Journal of Membrane Science 249, p235-243, (2005).
[https://doi.org/10.1016/j.memsci.2004.10.040]

- T. Li, P. Yu, and Y Luo, “Preparation and properties of hydrophobic poly (vinylidene fluoride)-SiO2 mixed matrix membranes for dissolved oxygen removal from water”, Journal of Applied Polymer Science, p40430-40437, (2014).
- P.W. Heo, and I.S. Park, “Separation characteristics of dissolved gases from water using a polypropylene hollow fiber membrane module with high surface area”, World Academy of Science, Engineering and Technology, 8(7), p1266-1269, (2014).
-
Velianti, S.B. Park, and P. W. Heo, “The enhancement of oxygen separation from the air and water using poly (vinylidene fluoride) membrane modified with superparamagnetic particles”, Journal of Membrane Science 466, p274-280, (2014).
[https://doi.org/10.1016/j.memsci.2014.04.043]