The effect of cathodic protection system by means of zinc sacrificial anode on pier in Korea
Copyright © The Korean Society of Marine Engineering
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
This study has been conducted to confirm the effect of sacrificial anode cathodic protection system for 90 days to protect corrosion on pier that is located in Korea. The cathodically protected structure was a slab and a pile cap. Before the construction of cathodic protection system, the macrography was carried out. As a result of the macrography, many corrosion traces were confirmed in this structure. The trace was mainly focused on joint and zones that concrete cover was eliminated. To apply the cathodic protection system, many onsite techniques have been adopted. In addition, to confirm the inner state of steel in concrete properly, a corrosion monitoring sensor (DMS-100, Conclinic Co. Ltd) has been applied. Test factors were corrosion & cathodic protection potential, 4 hour depolarization potential, resistivity and current density. After 90 days from the installation of cathodic protection system, it could confirm that proper corrosion protection effect was obtained by considering the result of tests.
Keywords:
Cathodic protection, Sacrificial anode, Reinforced concrete, Corrosion, Potential1. Introduction
Corrosion, the degradation of metal, is an electrochemical reaction between a metal or metal alloy and its environment [1][2]. In the case of harsh corrosion conditions such as seawater, most materials have a tendency to be severely corroded before its life expectancy. On the other hand, in the case of reinforced concrete, corrosion is hard to occur. This is because concrete is alkaline with pH of 12-13, which is one of the optimal conditions for anti-corrosion of steel in concrete [3]-[10].
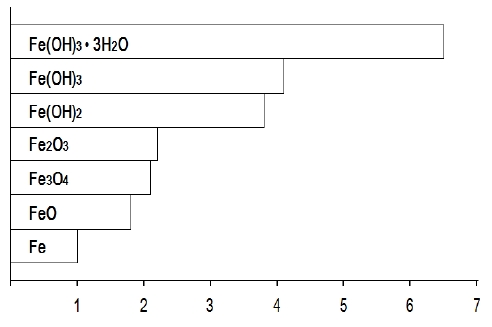
However, steel in concrete can be corroded in some critical condition, with reducing the pH of concrete. The major elements on corrosion of steel in concrete are chloride ion and carbon dioxide. Specifically, chloride ions, which are contained in seawater and utilizes as de-icing salt, are not consumed in the electrochemical reaction. In other words, it acts as a catalyst in the corrosion reaction. In addition, during the reaction, hydrochloric acid is made with reducing pH of concrete locally [11]-[15]. Most critical aspects in corrosion is an increase in the volume of steel. As the reactions are processed, the volume of the steel/concrete interface increases up to 6-7 times as shown in Figure 1[3], which contributes to the cracking and spalling of concrete structures.
To protect corrosion of steel in concrete, many advanced ways have been developed. Among these methods, cathodic protection (CP) has been applied in many industries, and now it is considered as one of the proven technologies in corrosion protection [16][17].
Particularly, sacrificial anode CP system is a very attractive method considering its inherent simplicity and low maintenance requirement [18][19]. There have been conducting many laboratory studies related to sacrificial anode CP system. However, onsite environments contain many variables in order to apply the system. Thus, in this study, the construction of CP system by means of a zinc sacrificial anode was carried out to obtain the onsite data for CP system in coal pier in Korea.
2. Experimental method
2.1 Construction of cathodic protection system
Figure 2 shows positions where CP systems were installed. Places were a slab and a pile cap, and the positions were selected after the macrography. Traces of severe corrosion damage were found in these places.
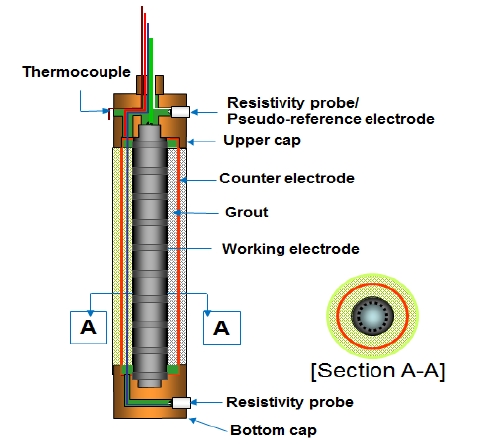
Figure 3 is the schematic drawing of the proposed sensor in this study for corrosion monitoring in concrete and all inside space of the sensor was filled with epoxy to prevent concrete specimens from any detrimental factors like sea water. In addition, described earlier, the corrosion monitoring sensors, DMS-100, were installed. The sensor can measure various factors such as potential, current density, corrosion rate, resistivity, and temperature.
The sensor was installed to enhance the accuracy of tests by comparing with the results of steel in concrete. Each two sensors were installed for comparison. The sensor has guaranteed its performance in many laboratory and onsite studies [14][15].
Figure 4 and 5 show the process of construction of CP system. Firstly, the surface treatment of damaged areas was carried out for the purpose of an increase in efficacy of anodes. After the surface treatment, anode and rebar was electrically connected. Lastly, zinc anodes with a mesh type were installed with the consideration for dimensions, and the places were cast in concrete with proper concrete cover depth of 3cm. After construction of CP, damage spaces were recovered. As shown in Figure 4 (d) and 5 (d), structures were well restored with a clean surface.
2.2 Procedures and test factors
Experiments were carried out for 90 days. During the period, three times of measurements were conducted. First measurement was implemented after installing CP system. Second measurement was conducted after a month from initial measurement. Last measurement was conducted after three month from the initial measurement. Test factors were corrosion & CP potential, 4 hour depolarization potential, current density, and concrete resistivity.
3. Results and discussion
3.1 Potential variations
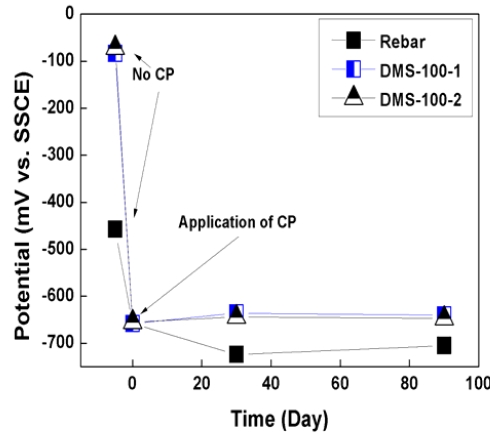
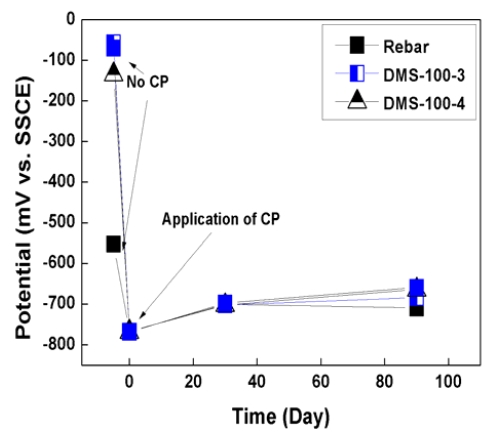
Figure 6 and 7 show the potential variations of rebar for 90 days. As shown in graphs, initial rebar potential of the slab was -457mV vs. SSCE and that of the pile cap was -553mV vs. SSCE. According to ASTM C 876 [20], if potential is lower than -400mV vs. SSCE, rebar is regarded as severely corroded state. The state of rebar was very critical for corrosion. However, in the case of the DMS-100 sensors, sensors are regarded as passive state with a higher potential range of 50~100mV vs. SSCE.
When CP was applied, potential both rebar and sensors was decreased to -750mV ~ -650mV vs. SSCE, which is sustained for more than 90 days. In the case of sensors, higher potential variations were confirmed than rebar because sensors did not be corroded.
3.2 Current density and resistivity variations
Current density can be a key factor in measuring CP state of rebar. Through a number of laboratory and field experiment, the required current densities for CP was 10-20mA/m2, which might extend the service life for the already corroded steel in concrete. It is much higher than that for no corrosion steel in concrete (2mA/m2) [21][22].
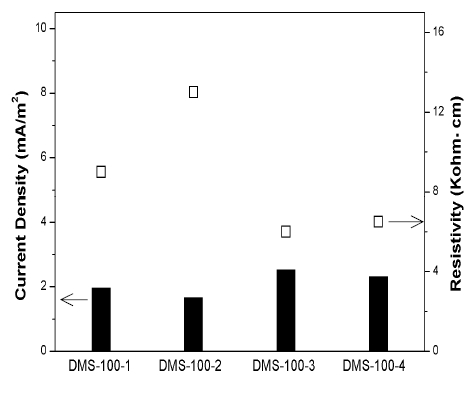
Figure 8 shows the CP current density variation of DMS-100 sensors after applying CP for 30 days. The values of current density were 1.8 ~ 2.5mA/m2. Considering the sensor was no corroded, the zinc anode can supply enough CP current. However, it was hard to decide the steel's CP condition by solely checking the result of sensors' current density because steel in concrete has been corroded for a long time, which was different condition with sensors.
In addition, current density has a tendency to be in inverse proportion to resistivity. This is because resistivity plays a key factor to decide the exchanging rate of ions.
3.3 Depolarization potential variations
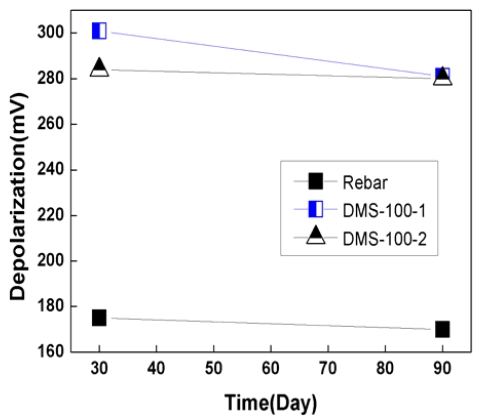
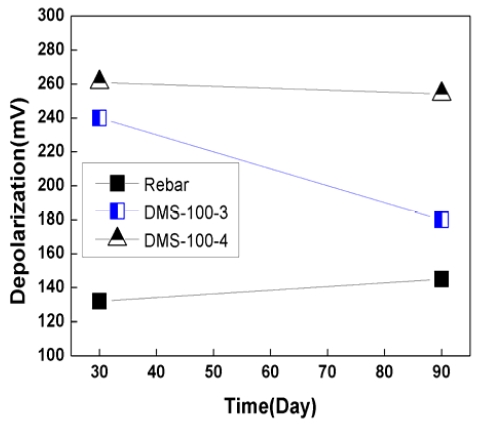
Figure 9 and 10 show depolarization potential variation for 90 days. Depolarization potential is normally measured by disconnecting anode from rebar for 4 hours or 24 hours. In this case, the 100mV depolarization criterion for 4 hours was utilized to check the effectiveness of sacrificial anode CP system.
As shown in Figure 9 and 10, the depolarization of rebar was 135mV ~ 180mV, which met the 100mV depolarization criterion.
In the case of DMS-100 sensor, depolarization potential were 200mV ~ 315mV, which was higher than rebar. Depolarization potential of rebar and sensors had a tendency to decrease with time. This was thought that due to the dry of concrete, concrete resistivity was increase with time, which has reduced CP effects.
4. Conclusions
This study described the effect of sacrificial anode CP system for onsite concrete structures. The sacrificial anode CP system can be a useful way to protect corrosion in marine concrete structure. The major results are summarized as follows:
1. After removing concrete and pilling with new concrete with CP system, overall surface condition is good.
2. Initial rebar potential both slab and pile cap was lower than -400mV vs. SSCE. Accodring to ASTM C876, the potential range was regarded as severe corrosion section.
3. After CP was connected with rebar, potential was dec reased down to -750mV ~ 650mV vs. SSCE, which showed higher potential variation than 300mV.
4. Current density of sensor was 1.8 ~ 2.5mA/m2. It was enough to protect the sensors; however, it was hard to decide on whether rebar was cathodically protected be cause the state of rebar was different from sensor.
5. Depolarization potential was more than 100mV in both re bar and sensors, which met the NACE Standard SP0290.
References
-
K. M. Moon, Y. H. Kim, S. Y. Lee, J. D. Kim, M. H. Lee, and J. G. Kim, “Electrochemical evaluation on corrosion property of welding zone of 22APU stainless steel”, Journal of the Korean Society of Marine Engineering, 33(8), p1162-1169, (2009).
[https://doi.org/10.5916/jkosme.2009.33.8.1162]

- D. A. Jones, Principles and Prevention of Corrosion, 2nd Edition, Upper Saddle River, New Jersey, the US: Prentice-Hall, (1996).
- J. P. Broomfield, Corrosion of Steel in Concrete, 2nd Edition, London and New York, the US: Taylor & Francis, (2007).
-
M. Saleem, M. Shameem, S.E. Hussain, and M. Maslehuddin, “Effect of moisture, chloride and sulphate contamination on the electrical resistivity of portland cement concrete”, Journal of Construction and Building Materials, 10(3), p209-210, (1996).
[https://doi.org/10.1016/0950-0618(95)00078-X]

- B. Sederholm, and J. Almqvist, “Corrosion properties of stainless steels as reinforcement in concrete in swedish outdoor environment”, Proceedings of CORROSION, 203, p1-2, (2009).
-
G. Blancoa, A. Bautista, and H. Takenoutib, “EIS study of passivation of austenitic and duplex stainless steels reinforcements in simulated pore solutions”, Journal of Cement and Concrete Composites, 28(3), p212-213, (2006).
[https://doi.org/10.1016/j.cemconcomp.2006.01.012]

- J. M. Ha, C. K. Jin, and J. A. Jeong, “Cathodic protection behavior of coastal bridge structure with sacrificial anode cathodic protection system”, Journal of Corrosion Science and Technology, 11(6), p242-243, (2012).
-
J. A. Jeong, C. K. Jin, and W. S. Chung, “Tidal water effect on the hybrid cathodic protection systems for marine concrete structures”, Journal of Advanced Concrete Technology, 10(1), p389-390, (2012).
[https://doi.org/10.3151/jact.10.389]

-
J. A. Jeong, and C. K. Jin, “Characteristic of CP potential for concrete pile specimen with hybrid CP”, Journal of Advanced Material Research, 685(3), p3-7, (2013).
[https://doi.org/10.4028/www.scientific.net/AMR.685.3]

-
J. A. Jeong, and C. K. Jin, “The effect of temperature and relative humidity on concrete slab specimens with impressed current cathodic protection system”, Journal of Korean Society of Marine Engineering, 37(3), p260-262, (2013).
[https://doi.org/10.5916/jkosme.2013.37.3.260]

- C. K. Jin, J. A. Jeong, and E. J. Kyeong, “A study on the corrosion monitoring of multi-functional sensors for reinforced concrete structures : part 1”, Journal of Corrosion Science and Technology, 11(6), p270-274, (2012).
-
D. Trejo, and P. J. Monteiro, “Corrosion performance of conventional (ASTM A615) and low-alloy (ASTM A706) reinforcing bars embedded in concrete and exposed to chloride environments”, Journal of Cement and Concrete Research, 35(3), p562-563, (2005).
[https://doi.org/10.1016/j.cemconres.2004.06.004]

- H. Yu, X. Shi, W. H. Hartt, and B. Lu, “Laboratory investigation of reinforcement corrosion initiation and chloride threshold content for self-compacting concrete”, Journal of Cement and Concrete Research, 40(10), p1507-1508, (2010).
- J. A. Jeong, C. K. Jin, E. J. Kyoung, G. J. Jo, and K. J. Kim, “An introduction of a multi-functional sensor for reinforced concrete structure”, Proceedings of the 36th KOSME Fall conference, p99, (2012).
-
J. A. Jeong, and C. K. Jin, “Three year performance of sacrificial anode cathodic protection system in the reinforced concrete bridge structures”, Journal of Advanced Materials Research, 753-755, p467-475, (2013).
[https://doi.org/10.4028/www.scientific.net/AMR.753-755.467]

-
C. Christodoulou, G. Glass, J. Webb, S. Austin, and C. Goodier, “Assessing the long term benefits of impressed current cathodic protection”, Journal of Corrosion Science, 52(8), p2671-2679, (2010).
[https://doi.org/10.1016/j.corsci.2010.04.018]

-
L. Bertolini, and E. Redaelli, “Throwing power of cathodic prevention applied by means of sacrificial anode to partially submerged marine reinforced concrete piles : results of numerical simulations”, Journal of Corrosion Science, 51(9), p2218-2230, (2009).
[https://doi.org/10.1016/j.corsci.2009.06.012]

-
A. A. Sagues, and R. G. Powers, “Sprayed-zinc sacrificial anodes for reinforced concrete in marine service”, Journal of Corrosion, 52(7), p508-522, (1996).
[https://doi.org/10.5006/1.3292141]

- M. Funahashi, and W. T. Young, “Three year performance of aluminum alloy galvanic cathodic protection system”, Proceedings of CORROSION, (550), p1-16, (1999).
- American Society for Testing and Materials, “Standard test method for corrosion potentials of uncoated reinforcing steel in concrete”, The US, ASTM C876, (2009).
- L. Bertolini, F. Bolzoni, P. Pedeferri, and T. Pastore, “Three year tests on cathodic prevention of reinforced concrete structures”, Proceedings of CORROSION, (244), p1-16, (1997).
-
L Bertolini, F Bolzoni, A Cigada, T Pastore, and P Pedeferri, “Cathodic protection of new and old reinforced concrete structures”, Journal of Corrosion Science, 35(5-8), p1633-1639, (1993).
[https://doi.org/10.1016/0010-938X(93)90393-U]